The annual flu epidemics caused by influenza viruses, especially the influenza A virus, affect about 10–20% of the world’s population each season. This highly contagious illness can trigger serious complications and can still lead at times to death, even today. Scientists have been working for a while with the M2 proton channel from the influenza A virus, which is one of nature’s smallest proton-selective channels and also a drug target for anti-influenza medications. Now, for the first time, they were able to gain insights at high resolution into the M2 channel.
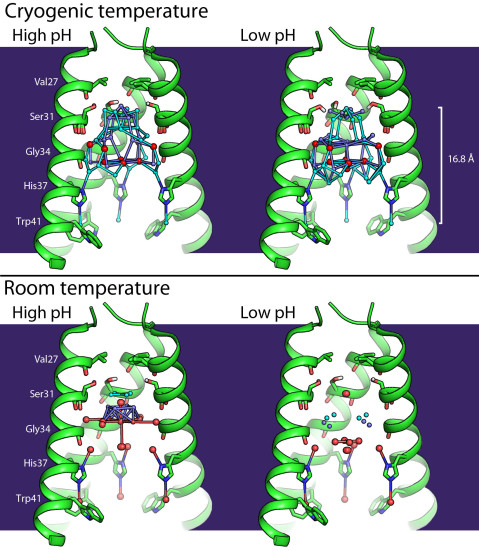
With the help of crystallographic techniques at Beamline 8.3.1, the crystallized M2 proteins revealed their inner network of water molecules, ordered inside the channel, that could contribute to the protein’s proton-transport mechanism. The protein crystals were diffracted twice, under cryogenic conditions and at room temperature, to make sure that ice (an artificially ordered water network) hadn’t been created within the channel and to avoid radiation damage at room temperature.
A better understanding of how M2 stabilizes and conducts protons may help in designing better anti-influenza medications that can inhibit the M2 proton channel and thus impede the influenza A virus.
Work performed at ALS Beamline 8.3.1.
Jessica L. Thomaston, Mercedes Alfonso-Prieto, Rahel A. Woldeyes, James S. Fraser, Michael L. Klein, Giacomo Fiorin, and William F. DeGrado, “High-resolution structures of the M2 channel from influenza A virus reveal dynamic pathways for proton stabilization and transduction,” PNAS 112, 14260 (2015).