As the number of electric vehicles on the road increases, there is a growing need to improve the capacity, lifespan, and safety of lithium-ion batteries. In particular, the bottleneck lies in the oxide-based positive electrode, or cathode, which becomes unstable when charged to high voltages. LiNixCoyMn1-x-yO2 (NCM) is one of the most commonly used commercial cathode materials in today’s electric vehicles due to its high energy density—which offers the potential for longer driving ranges and quick acceleration—yet it suffers from stability issues.
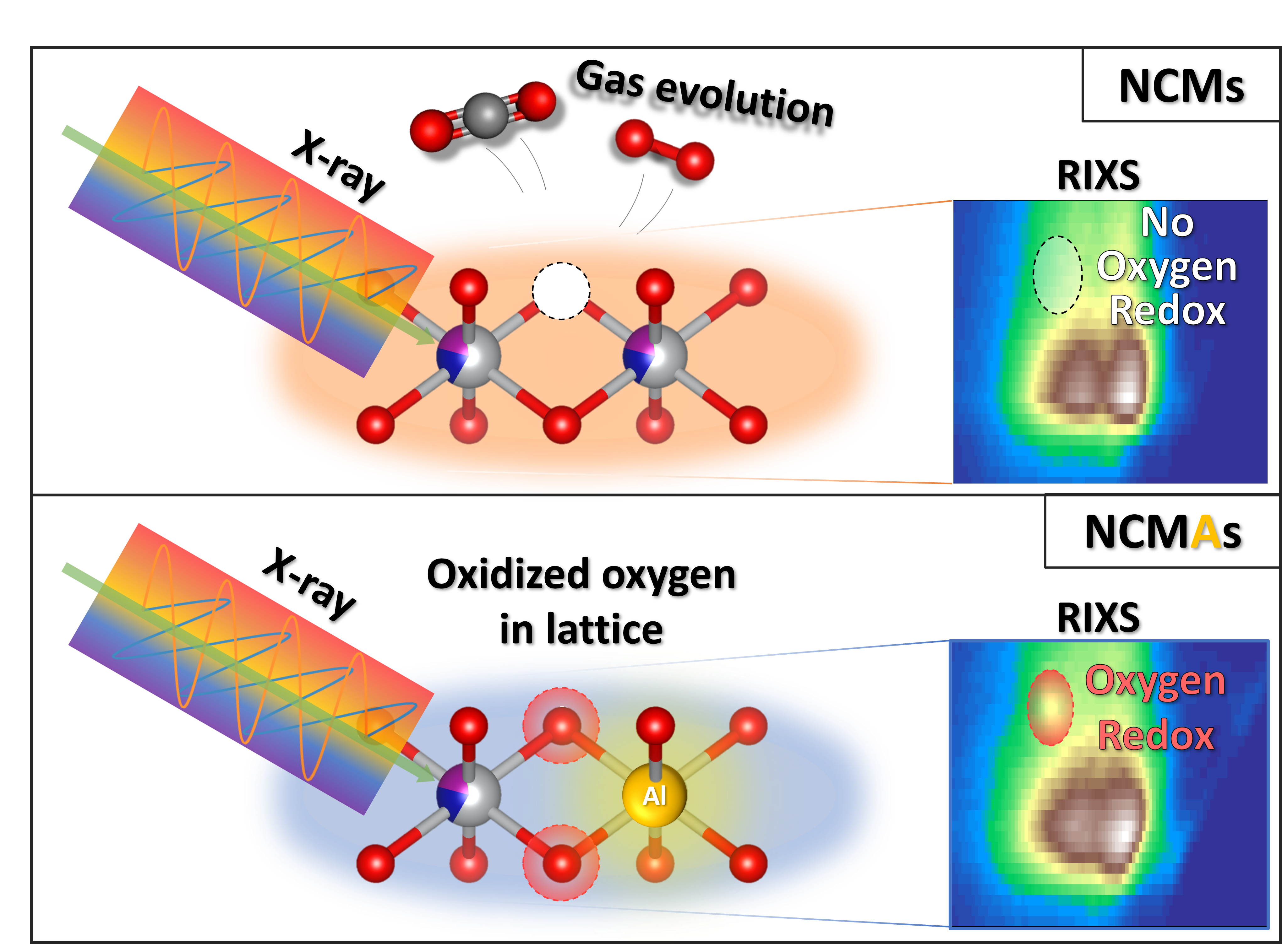
While oxygen reduction and oxidation (redox) reactions can boost energy density at high voltages, the oxidation of oxygen often triggers chemical reactions that degrade the material over time, reducing battery life and performance. In a study published recently in Energy and Environmental Science, researchers investigated this tradeoff by comparing NCM materials from LG Energy Solution, one of the world’s largest battery suppliers, with and without aluminum doping, which is commonly used in industry to improve stability.
“We were able to associate oxygen redox activities with structural and electrochemical properties of the electrodes and reveal the role of aluminum doping in NCM materials,” said Gi-Hyeok Lee, postdoctoral researcher at the Advanced Light Source (ALS) and first author of the study.
To understand the role of oxygen in these materials, the team used soft x-ray resonant inelastic x-ray scattering (RIXS) at ALS Beamline 8.0.1 to identify distinct oxygen redox activities within the cathode material. Meanwhile, operando electrochemical mass spectrometry (OEMS) at University of California, Berkeley was used to track oxygen release from the material and electrolyte-material interface. Surprisingly, the aluminum-doped NCM materials show increased oxygen redox activity in RIXS, yet less oxygen release in OEMS, in contrast with the common belief that more strongly oxidized oxygen triggers more oxygen loss. This suggests that aluminum doping enhances oxygen redox reactions but reduces the detrimental oxygen loss. Further insights from neutron diffraction experiments linked this oxygen behavior to structural changes in the material, indicating that managing oxygen redox reactions at high voltages could mitigate irreversible structural evolutions in NCM materials.
“Now we understand the irreversibility directly from its origin, instead of just seeing the resultant structural changes,” said Lee. This research clarifies the oxygen redox activities during high voltage cycling of commercial NCM materials and elucidates the impact of dopants on structural and electrochemical stability, helping researchers further optimize commercially viable materials for high-energy-density batteries.
G.-H. Lee, S. Lee, J. Zhang, B. L. D. Rinkel, M. J. Crafton, Z. Zhuo, Y. Choi, J. Li, J. Yang, J. W. Heo, B. Park, B. D. McCloskey, M. Avdeev, W. Yang, and Y.-M. Kang, “Oxygen redox activities governing high-voltage charging reversibility of Ni-rich layered cathodes,” Energy Environ. Sci. 17, 9154 (2024). doi:10.1039/D4EE03832K.