SCIENTIFIC ACHIEVEMENT
A bimetallic material (Pd-Ni) produces hydrogen-active nanopockets that improve the efficiency and lower the cost of hydrogen storage systems.
SIGNIFICANCE AND IMPACT
Mechanistic understanding of a Pd-Ni bimetallic system paves the way to design cost-effective hydrogen storage, opening new opportunities to develop reliable energy technologies for the energy industry.
Hydrogen energy potential
Hydrogen is one of the most promising new fuel sources. This renewable resource has three times more energy per unit mass than gasoline. Despite its promise, hydrogen storage, particularly for light-duty vehicles, has remained elusive, hindering commercial applications.
Hydrogen storage requires a material that can adsorb hydrogen in a quasi-molecular bonding process. Palladium (Pd), a chemical element with a high affinity for hydrogen, can form a stable Pd−H bond; however, pure Pd nanostructures are insufficient for large-scale hydrogen storage.
To improve hydrogen storage capacity, bimetallic nanostructures allow for the creation of new atomic sites for hydrogen adsorption, which may be adjusted through stoichiometry.
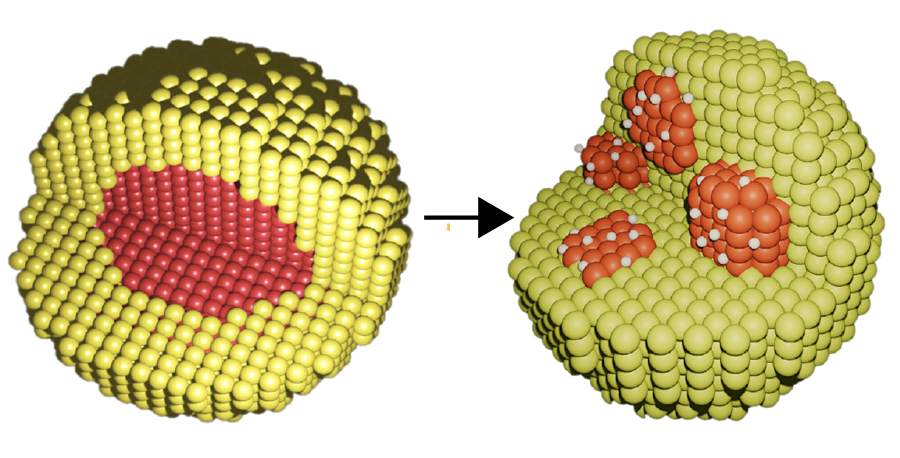
In this study, the research team explored a mixture of cost-effective nickel oxide (NiO) and Pd in the formation of Pd-NiO nanoparticles at different concentrations at the Advanced Light Source (ALS) at Lawrence Berkeley National Laboratory. The pristine, as-prepared nanoparticles consisted of a Pd core surrounded by a NiO shell. The research team investigated the atomic interactions that occur inside the nanoparticles to understand how the structure and chemistry of the bimetallic nanoparticles change in the presence of hydrogen.
Shedding light on an energy question
The team used ambient-pressure x-ray photoelectron spectroscopy (APXPS) and grazing-incidence x-ray scattering (AP-GIXS) at the APPEXS endstation at Beamline 11.0.2. At this endstation, the researchers gathered simultaneous measurements to determine the chemical composition, hydrogen bonding, and morphological changes under operating temperature and ambient-pressure conditions.
They used APXPS at Beamline 9.3.2 to probe the chemistry of samples. These measurements were complemented by transmission electron microscopy and electron energy loss spectroscopy at the National Center for Electron Microscopy at the Molecular Foundry, which generated nanoscale insights into the particle morphology and chemistry.
The combined techniques revealed the complex interactions taking place at the atomic level on the bimetallic nanoparticles. During hydrogen storage, NiO acts as a buffer as the Pd core disassembles, forming numerous Pd-rich pockets. As hydrogen binds to the Pd, the Pd pockets increase their surface area available for hydrogen storage. The team also found that nanoparticles with a lower concentration of Pd produced more pockets, thereby increasing hydrogen storage capacity while lowering the total cost.
New opportunities to increase hydrogen storage
By using in situ x-ray techniques, the research team gained a comprehensive understanding of how these nanoparticles change morphologically and chemically at the atomic level. Their findings present a new cost-effective solution for hydrogen storage that could drive development of energy technologies. Future research could focus on applying similar methodology to bimetallic nanoparticle systems of different compositions and morphologies to evaluate the efficiency and cost effectiveness of potential hydrogen storage solutions.

Contacts: Lívia Matte and Fabiano Bernardi
Researchers: L. P. Matte (Instituto de Física, Universidade Federal do Rio Grande do Sul, Brazil and ALS); A. S. Thill, F. Poletto, and F. Bernardi (Instituto de Física, Universidade Federal do Rio Grande do Sul, Brazil); M. Jaugstetter and T. P. Mishra (Berkeley Lab); C. Escudero (ALBA Synchrotron Light Source); and G. Conti and S. Nemsak (ALS, University of California, Davis).
Funding: Research Support Foundation of the State of Rio Grande do Sul (FAPERGS), the Brazilian National Council for Scientific and Technological Development (CNPq), the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES), the Spanish Ministry of Science and Innovation (MICINN-FEDER), and US Department of Energy, Office of Science, Basic Energy Sciences program (DOE BES), Catalysis Research Program. Operation of the ALS and Molecular Foundry are supported by DOE BES.
Publication: L.P. Matte, M. Jaugstetter, A.S. Thill, T.P. Mishra, C. Escudero, G. Conti, F. Poletto, S. Nemsak, and F. Bernardi, “Direct Observation of Phase Change Accommodating Hydrogen Uptake in Bimetallic Nanoparticles,” ACS Nano 19, 10 (2025), doi: 10.1021/acsnano.4c18013.
ALS SCIENCE HIGHLIGHT #524