
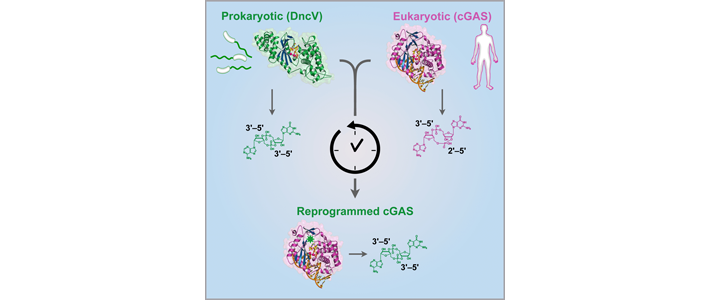
The human immune system deploys an array of sensors to detect invading pathogens like viruses. One of these sensors, the human enzyme cGAS, detects invading DNA and initiates an antiviral response by producing a unique signaling molecule known as a cyclic dinucleotide. Cyclic dinucleotide signals are also found in bacteria, but previously no direct connection existed between the signaling pathways in bacteria and human immunity. Jennifer Doudna, James Berger, and colleagues now show that a bacterial signaling protein critical for pathogenesis in Vibrio cholerae is actually a homolog of human cGAS. These results reveal a surprising evolutionary connection between bacterial signaling and human innate immunity.
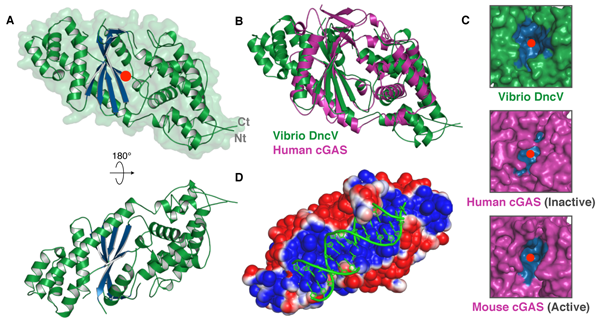
(A) Crystal structure of Vibrio cholerae DncV. a helices are colored in green, b strands forming the enzyme core are in blue, and a red circle marks the active site. (B) Superposition of the crystal structures of DncV (green) and human cGAS (purple) (PDB 4KM5). (C) Surface view representation of the DncV (green), apo human cGAS (purple, ‘‘inactive’’), and dsDNA- bound mouse cGAS (purple, ‘‘active’’) active site substrate channels (mouse cGAS PDB 4K98). Nucleotide substrate binding pockets are colored in blue, and the active site is marked by a red circle. (D) Superposition of an electrostatic surface view of DncV colored by charge (positive, blue and negative, red) and cGAS-bound dsDNA (green). The structures of DncV and dsDNA-bound mouse cGAS were superposed without manual fitting of the dsDNA ligand.
Additionally, comparison of structures of the two enzymes reveals a mechanism to explain chemical differences between bacterial and human cyclic dinucleotide signals, and explains how human cGAS produces a unique molecule to warn of invading bacterial and viral pathogens.
Work performed on ALS Beamline 8.3.1.
Citation: Philip J. Kranzusch, Amy S.Y. Lee,Stephen C. Wilson, Mikhail S. Solovykh, Russell E. Vance, James M. Berger, and Jennifer A. Doudna, “Structure-Guided Reprogramming of Human cGAS Dinucleotide Linkage Specificity,” Cell 158, 1011 (2014), doi:http://dx.doi.org/10.1016/j.cell.2014.07.028.