According to the Centers for Disease Control and Prevention, inflammatory bowel disease (IBD) affects more than three million people across the United States, costing the nation’s healthcare system about $8.5 billion in 2018. IBD occurs when the body’s immune system mistakenly attacks healthy bowel tissue, leading to inflammation and damage.
The exact cause of IBD is unknown, but the disease is characterized by long-term inflammation. While many factors can influence inflammation, interleukin-10 (IL-10), a specialized protein, plays a role in regulating the immune response in the human body and has the potential to treat inflammation-related conditions. Unfortunately, wild-type IL-10 is complicated. It possesses a dual nature and can initiate both pro- and anti-inflammatory pathways. It is also ephemeral, only lasting three hours in the human body.
“Natural proteins can be problematic as biotherapeutics, because these compounds often have a limited half-life and toxicity,” said Glen Spraggon, executive director of structure bioinformatics and data science at the Novartis Biologics Research Center and senior author on the study. “We decided to try to combine the positive properties of antibodies—half-life and good manufacturability—with the functional properties of IL-10.”
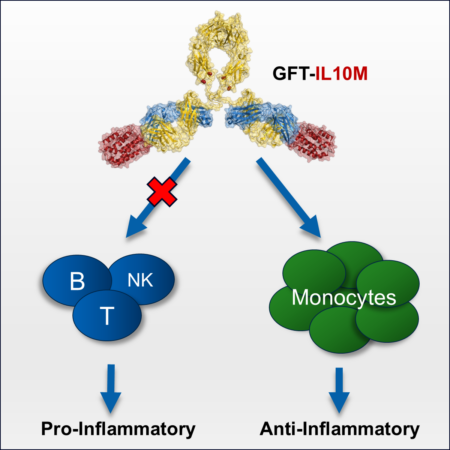
Spraggon and his colleagues grafted a modified IL-10 protein into the complementarity determining regions (CDRs) of an antibody. Through this process, they engineered six graft variants. One graft (GFT-IL10M) had the desired properties, but the actual molecular structure of the design remained unclear. The joints in the graft connecting the antibody and IL-10 are incredibly flexible. At the ALS, the team crystallized the engineered sample and analyzed it at Beamline 5.0.3. They used the diffraction data to define the three-dimensional structure of the IL-10 fusion protein at the atomic level.
“We love to have a visual to understand the molecular structure of what we have actually created in the lab,” said Spraggon. “This structure confirmed how, with this fusion approach, we largely change the signaling profile of the molecule, biasing it away from its pro-inflammatory nature towards anti-inflammatory.”
GFT-IL10M optimizes the pharmacodynamic and pharmacokinetic properties relative to wild-type IL-10, offering the potential of limiting the dose and dosing schedule of the therapeutic.
“The molecular insights developed with ALS data provide a template for future design, both with IL-10 and related cytokines facing the same challenges,” said Spraggon. “It also gives us additional insight into how complex the biology of this cytokine is.”
M. DiDonato, C. Turk Simpson, T. Vo, M. Knuth, B. Geierstanger, J. Jamontt, D.H. Jones, J.W. Fathman, D. DeLarosa, T. Junt, D. Picard, U. Sommer, M Bagger, E. Peters, S. Meeusen, and G. Spraggon, “A novel interleukin-10 antibody graft to treat inflammatory bowel disease,” Structure 33, P475-488.E7 (2025), doi:10.1016/j.str.2024.12.010.