Toyota has set ambitious targets for increased vehicle fuel efficiency and drive range, alongside decreased costs and component size. To achieve their goal of 1 million battery and fuel cell electric vehicles by 2030, they have prioritized fundamental research in fuel cell catalysis, tapping into resources in academia and at national labs. Recently, a collaboration between the Toyota Research Institute of North America and the University of Akron, enhanced by characterization at the Advanced Light Source (ALS), yielded a 40% improvement on existing catalysis technology. The finding was reported in the Journal of the American Chemical Society.
Fuel cells currently on the market rely on a series of slow oxidation-reduction reactions, requiring a platinum-based catalyst to speed up each step. As the process progresses, competition to use the platinum active site raises the energy required to continue the reaction. This leads to inefficiencies, even in electric vehicles currently on the market like the Toyota Mirai. Current technology has reached a plateau, motivating the Toyota Research Institute of North America (TRINA) to pursue improved catalysts. “Traditional platinum-alloy catalysts are all subject to a limit in their activity; overcoming this limit of the oxygen-reduction reaction is like mission impossible for the electrochemistry community,” explained Hongfei Jia, senior research manager for TRINA.
To circumvent some of the limitations of platinum, Jia worked with University of Akron Associate Professor Zhenmeng Peng. Xiaochen Shen, a graduate student in Peng’s lab, prepared fuel cell samples that introduced a second active site using tin oxide. Not only is tin oxide less costly than platinum, the energy required to use this active site is lower as well. Testing in the University of Akron and Toyota laboratories confirmed a 40% improvement in catalysis activity, as well as the presence of tin and oxygen in the sample, but not the precise mechanism by which they contribute to improved catalysis.
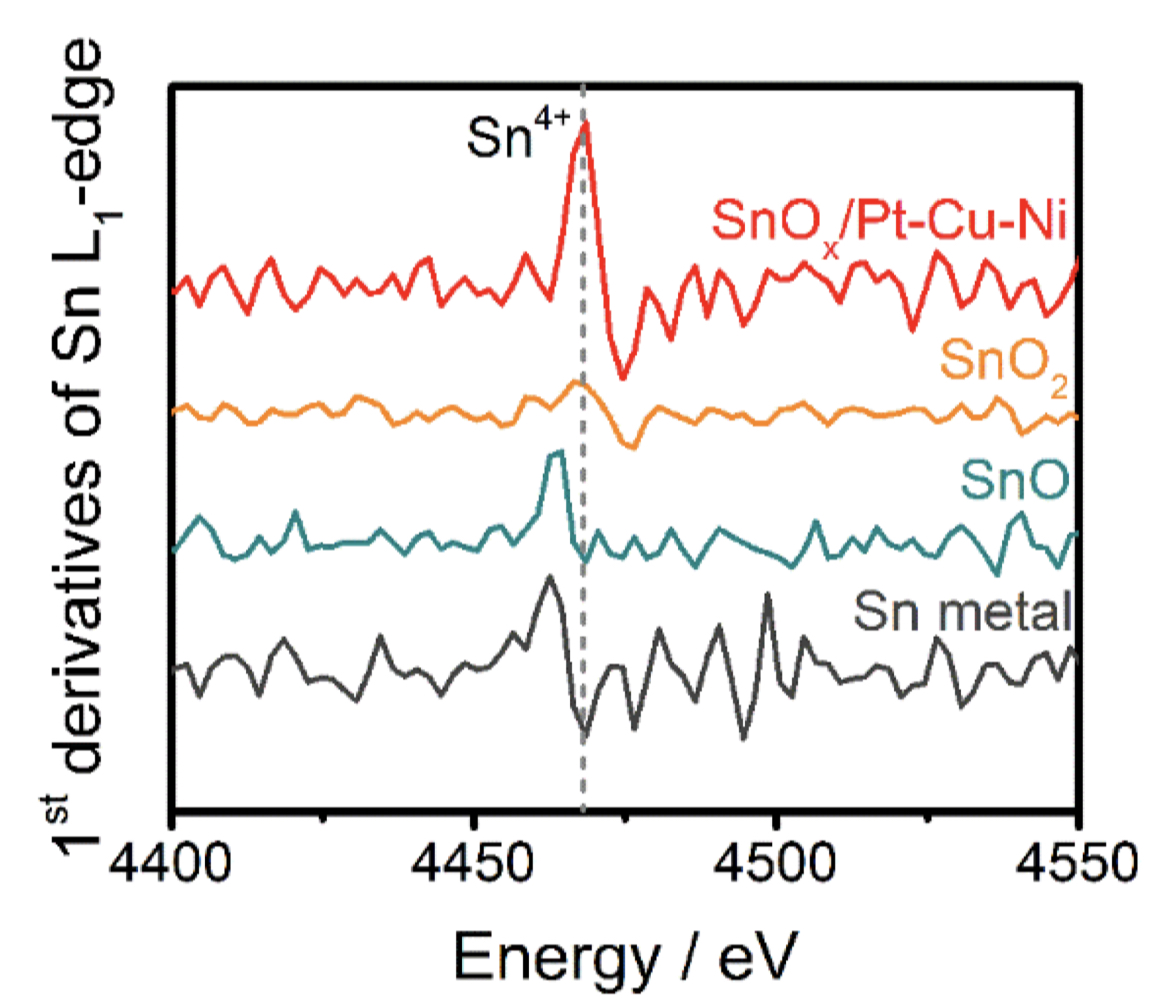
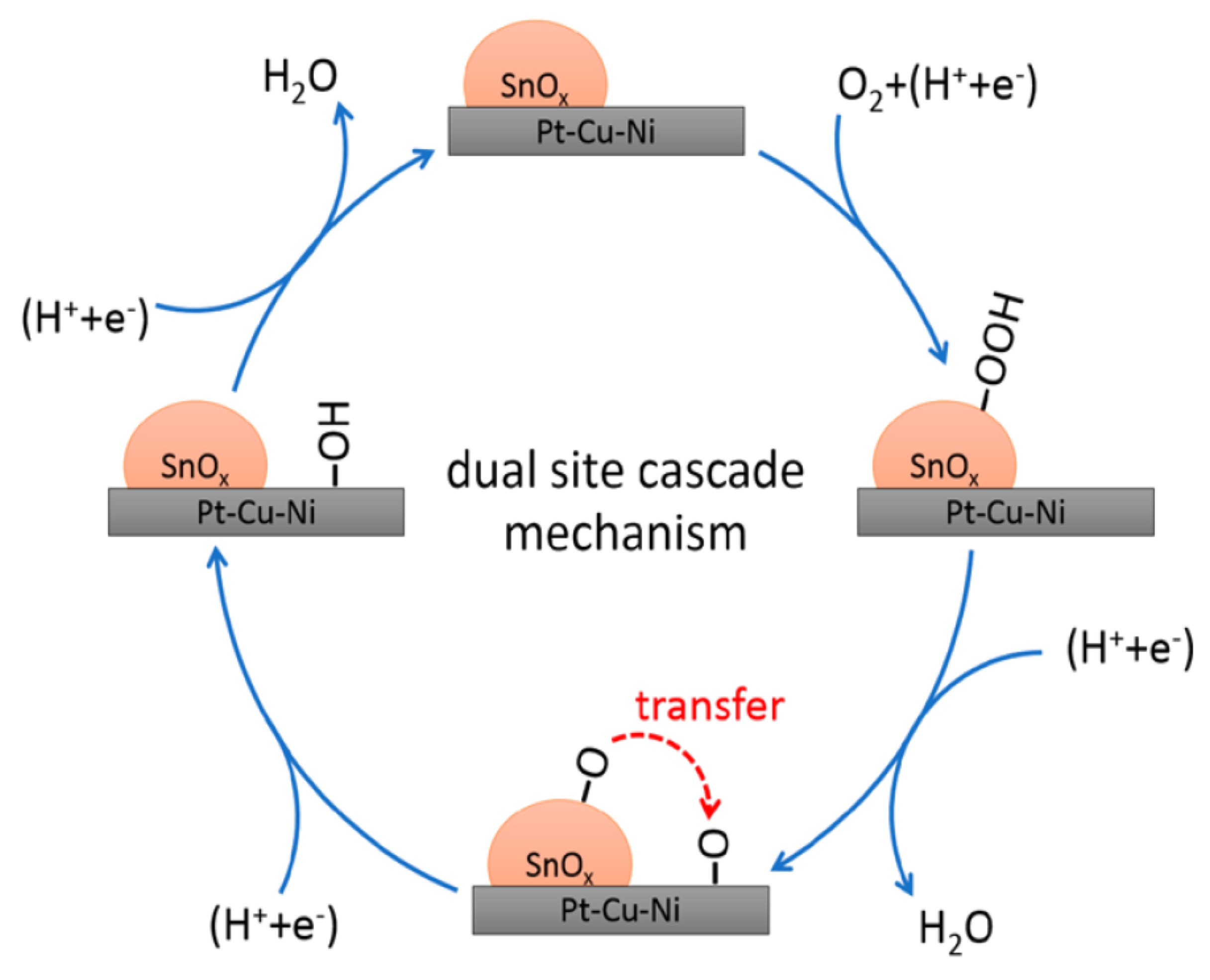
To clarify the behavior, “it was critical to confirm the structure and chemical valence of tin oxide,” Peng explained, so they turned to Senior Scientist Jinghua Guo at the ALS. At Beamlines 5.3.1 and 7.3.1, x-ray absorption near-edge structure (XANES) spectroscopy measurements revealed the oxidation state of tin in the samples to be Sn4+, allowing a deeper understanding of how tin oxide increases the efficiency of the catalyst. An initial oxidation-reduction reaction takes place in the tin oxide active site, which then promotes catalysis at the platinum active site, proceeding from a lower to a higher energy requirement. “The capabilities at the ALS really helped us confirm that we had the proposed structure; that was the initial proof of concept,” Jia said.
Jia views this discovery—and current products on the market—as only the beginning of a new era for fuel cell technology. “Long-term fuel cell research should be open. We hope the whole research and development community will join us to improve the technology—more researchers from national labs, industry, and academia. Everybody’s in the same boat.”
Contact: Hongfei Jia and Zhenmeng Peng
Researchers: X. Shen, Y. Pan, L. Yao, D. Wu, and Z. Peng (University of Akron); F. Yang (University of Akron and the Advanced Light Source); T. Nagai, L.Q. Zhou, and H. Jia (Toyota Research Institute of North America); Y.-S. Liu, J. Feng, and J. Guo (Advanced Light Source).
Funding: Toyota Research Institute of North America, the University of Akron, and the Ohio Research Scholars Program. Operation of the ALS is supported by the DOE Office of Science, Basic Energy Sciences Program.
Publication:X. Shen, T. Nagai, F. Yang, L. Q. Zhou, Y. Pan, L. Yao, D. Wu, Y.-S. Liu, J. Feng, J. Guo, H. Jia, and Z. Peng, “Dual-Site Cascade Oxygen Reduction Mechanism on SnOx/Pt–Cu–Ni for Promoting Reaction Kinetics,” J. Am. Chem. Soc. 141, 9463 (2019), doi:10.1021/jacs.9b02286.