Serotonin is a diminutive and deceptively simple-looking neurotransmitter molecule, yet a very complex “machinery” is required for neurotransmitter recognition, transmission, and recycling. The malfunctioning of this protein machinery can cause conditions such as depression, obsessive-compulsive disorder, aggression, anxiety, and Parkinson’s disease. In addition, drugs of addiction, such as methamphetamines, act on this system, causing serious damage and profoundly affecting the well-being of individuals. At the ALS, researchers were able to obtain x-ray crystallographic structures of the difficult-to-crystallize human serotonin transporter bound to two commonly prescribed antidepressant drug molecules. The resulting details about the transporter structure and mechanism will help in the design of new, more effective therapeutics for treating depression and anxiety.
Because serotonin has such a major impact on mood, proteins that bind and metabolize serotonin have been the target of intense pharmacological research. Drugs known as SSRIs—selective serotonin reuptake inhibitors—work by stopping serotonin from being reused by binding to the serotonin transporter (SERT) and blocking serotonin transport. Prozac, for example, is an antidepressant in this drug class, as are (S)-citalopram and paroxetine (commercial names are Lexapro and Paxil, respectively). These drugs work on the serotonin transporter, which is responsible for terminating serotonin signaling. When this transporter is blocked, serotonin accumulates in the synaptic space, effectively keeping the serotonin signal “on,” which can help alleviate symptoms of depression. Several illegal drugs, such as MDMA (“ecstasy”), also act on the serotonin transporter, but lead to a short-term feeling of euphoria and to permanent damage of neurons.
Here, the researchers focused on SERT, which belongs to a class of neurotransmitter transporter proteins that also transport dopamine and norepinephrine. SERT is a membrane protein and was extremely challenging to crystallize due to its poor stability after extraction from the membrane; however, the researchers were able to make SERT more tractable for crystallization by introducing a small number of mutations that improved stability, which in turn allowed successful collection of x-ray diffraction data at the Advanced Photon Source and at ALS Beamline 5.0.2 (part of the Berkeley Center for Structural Biology).

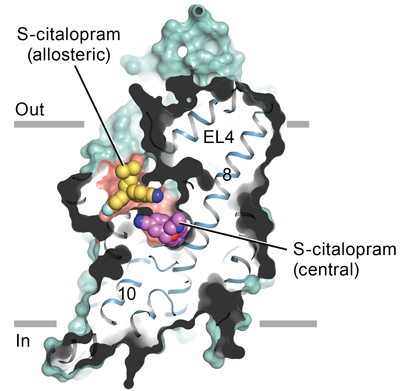
The study describes the high-resolution structure of SERT bound to the antidepressants (S)-citalopram and paroxetine, revealing many interesting characteristics of drug binding. Overall, SERT is embedded in the membrane of neurons by 12 membrane-spanning helices that house the central site that carries out the binding and transport of serotonin across the membrane. An opening, or “vestibule,” located in the transporter on the outside of the neuron allows access of serotonin and other ligands to the central binding site of SERT.
The antidepressants studied lock SERT in a configuration that is open to the outside of the neuron by physically binding to the central binding site, precluding serotonin binding and further motion. Interestingly, (S)-citalopram was also shown to bind to a site directly adjacent to the central binding site in the vestibule. Binding of SSRIs to the central site “wedges” the helices of SERT in an inactive configuration, and binding to the second site blocks drug release from the central site. Extensive interactions with specific amino-acid residues lining the binding site explain the selectivity of SERT for SSRIs. In addition, the research showed that the secondary binding site is extremely malleable, physically changing shape in response to ligands, and this plasticity could be exploited in future drug design work.
Contacts: Jonathan Coleman and Eric Gouaux
Research conducted by: J.A. Coleman and E.M. Green (Oregon Health & Science University) and E. Gouaux (Oregon Health & Science University and Howard Hughes Medical Institute).
Research funding: National Institutes of Health, Canadian Institute of Health Research, and Bernie and Jennifer LaCroute. Operation of the ALS is supported by the U.S. Department of Energy, Office of Basic Energy Sciences.
Publication about this research: J.A. Coleman, E.M. Green, and E. Gouaux, “X-ray structures and mechanism of the human serotonin transporter,” Nature 532, 334 (2016).
ALS SCIENCE HIGHLIGHT #336