We all want safer, longer-lasting batteries, but so far there is no “silver bullet” solution. Instead, it’s more a matter of optimizing a variety of interrelated parameters. For example, electrochemical (battery) cells that use aqueous electrolytes can be very safe, inexpensive, and environmentally friendly. However, their applications have been limited by their narrow voltage window of operation: about 1.23 V, too narrow to achieve high energy and power performance.
Now, researchers have demonstrated that an aqueous electrochemical system based on Na-ion insertion into Mn5O8 electrodes has a stable voltage window of 3.0 V, high energy and power performance, and nearly 100% coulombic efficiency and 85% energy efficiency after 25,000 charge-discharge cycles.
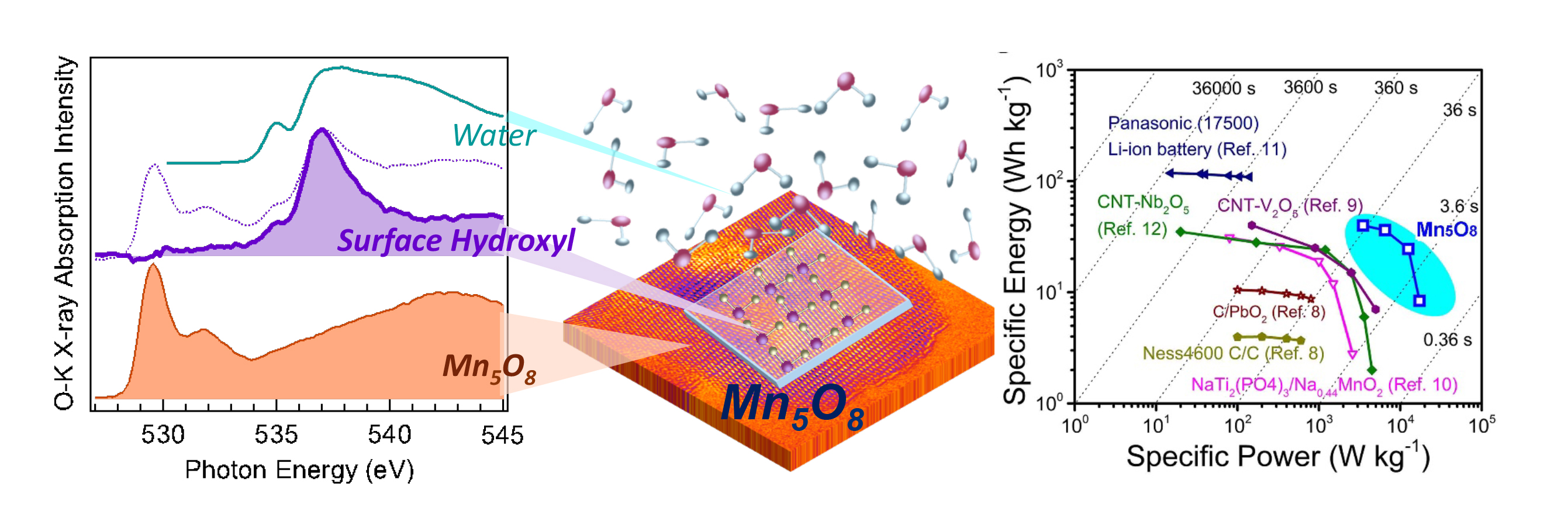
Mn5O8 is a unique layered material that forms an interesting solid–liquid “interphase” region with water. Utilizing soft x-ray spectroscopy at ALS Beamline 8.0.1, the researchers were able to identify the presence of adsorbed hydroxyl groups (OH) on the Mn5O8 surface. Furthermore, the hydroxyl groups were strikingly ordered, with perfectly aligned hydrogen bonds along the O–O direction, as in ice, but with a much longer distance between the O atoms.
It turns out that the interplay between the hydroxylated interphase and the unique bivalence (Mn2+2Mn4+3O8) structure of Mn5O8 greatly suppresses gas-evolution reactions, and as a result, expands the voltage window. These exciting results offer a new prospect for developing safe and environmentally benign electrode materials with performance comparable to non-aqueous Li-ion batteries.
Work performed at ALS Beamline 8.0.1.
X. Shan, D.S. Charles, Y. Lei, R. Qiao, G. Wang, W. Yang, M. Feygenson, D. Su, and X. Teng, “Bivalence Mn5O8 with hydroxylated interphase for high-voltage aqueous sodium-ion storage,” Nat. Commun. 7, 13370 (2016).