Comprising earth-abundant elements, cathodes made of magnesium/sulfur compounds could represent the next step in battery technology. However, despite being dendrite free and having a high theoretical energy density compared with lithium batteries, magnesium/sulfur batteries have suffered from high polarization and extremely limited recharging capabilities. To gain electrochemical insights into magnesium/sulfur batteries during charge–discharge cycles, researchers used the Advanced Light Source (ALS) to investigate and optimize battery chemistry.
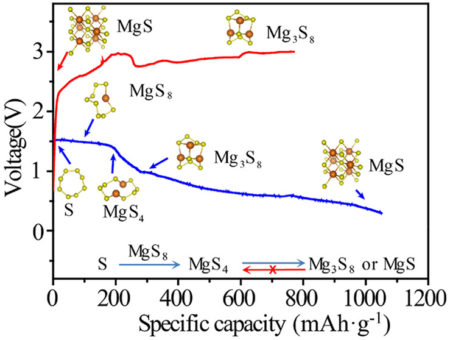
The in situ x-ray absorption spectroscopy (XAS) capabilities at ALS Beamlines 5.3.1 and 10.3.2 provided information on the oxidation state of sulfur under real operating conditions. The group found that the conversion of sulfur in the first discharging process was divided into three stages: formation of MgS8 and MgS4 at a fast reaction rate, reduction of MgS4 to Mg3S8, and a sluggish further reduction of Mg3S8 to MgS. The in situ XAS analysis revealed that Mg3S8 and MgS are more electrochemically inert and cannot revert to the active forms of sulfur, thereby dramatically reducing the battery’s cycling life.
After understanding the battery kinetics, the group sought a catalyst to drive the equilibrium toward the active forms of sulfur. Inspired by successes in lithium/sulfur and magnesium-ion batteries, they introduced a titanium-sulfide catalyst that activated the inert MgS and Mg3S8 species. With the catalyst, the battery had four times the discharge capacity after twenty cycles as previous magnesium/sulfur batteries.
These electrochemical insights pave the way to developing magnesium/sulfur batteries that are energy dense and can undergo many charge–discharge cycles.
Y. Xu, Y. Ye, S. Zhao, J. Feng, J. Li, H. Chen, A. Yang, F. Shi, L. Jia, Y. Wu, X. Yu, P.-A. Glans-Suzuki, Y. Cui, J. Guo, and Y. Zhang, “In situ x-ray absorption spectroscopic investigation of the capacity degradation mechanism in Mg/S batteries,”Nano Lett. 19, 5 (2019), doi:10.1021/acs.nanolett.8b05208.