SCIENTIFIC ACHIEVEMENT
Researchers uncovered the precise mechanism of hydrogen spillover (H2 splitting and migration) onto a catalytic surface by watching it happen under various conditions at the Advanced Light Source (ALS).
SIGNIFICANCE AND IMPACT
The research lays the foundation for designing more efficient catalysts and storage materials essential for next-generation hydrogen energy technologies.
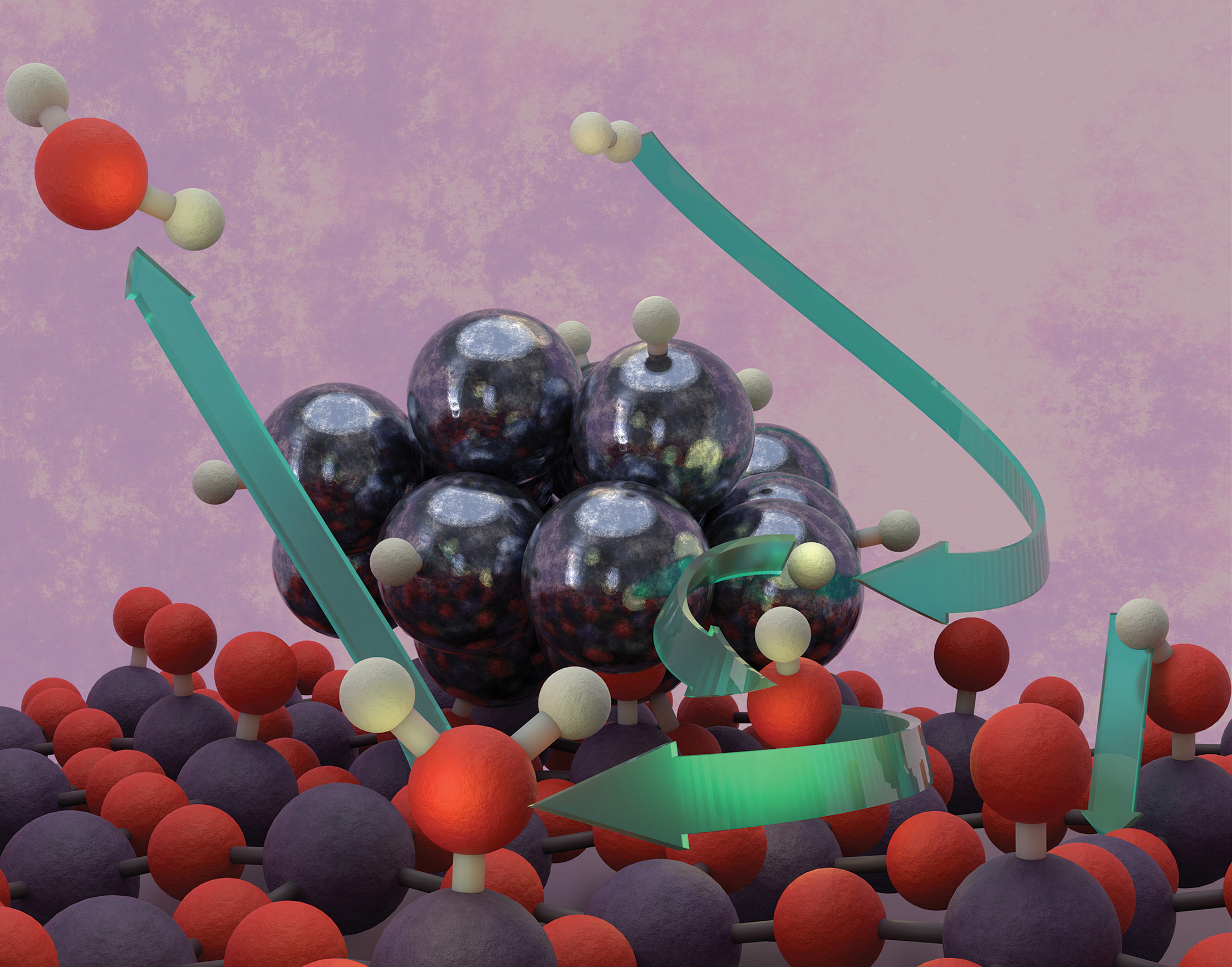
Hydrogen on the move
The splitting and migration of molecular hydrogen (H2) over a catalytic surface (a process known as “hydrogen spillover”) is a fundamental yet elusive phenomenon in catalysis that affects a wide range of uses, from hydrogenation (which can be used to upgrade or purify crude oil components) to energy storage (when bonded to a metal, hydrogen can be stored in the solid state). Despite its importance, direct experimental evidence capturing the real-time mechanistic steps of hydrogen spillover remains scarce.
In particular, tungsten oxide (WO3), a widely used catalytic material, exhibits dynamic interactions with hydrogen, yet the precise nature of these interactions has been a subject of long-standing debate, especially for distinguishing the chemical dynamics occurring on the surface from those in the bulk.
This research was driven by the need to resolve these ambiguities using ambient-pressure x-ray photoelectron spectroscopy (APXPS), which provides direct spectroscopic evidence of the spillover process as it unfolds. By integrating experimental observations with theoretical models, the researchers unlocked a comprehensive understanding of how hydrogen interacts with reducible oxide surfaces and influences their catalytic properties.
Operando APXPS at the ALS
This study focused on WO3 thin films “decorated” with Pt metal clusters that facilitate hydrogen activation and dissociation. To directly visualize the stepwise evolution of hydrogen spillover on WO3, the researchers employed APXPS at ALS Beamline 9.3.2, a technique pioneered at the ALS and uniquely suited for studying solid–gas interfaces in real time under realistic (“operando”) reaction conditions.
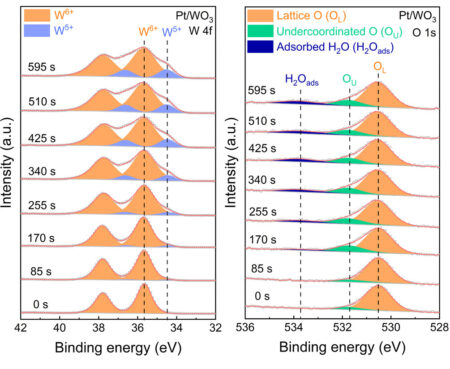
APXPS detected the oxidation states of tungsten and the presence of surface hydrogen species as the samples were exposed over time to hydrogen gas at various temperatures. The tunable incident photon energy allowed selective analysis of different elements (including differentiating between various hydrogen species—molecular, protonic, or hydride-like) at variable depths, enabling the researchers to track hydrogen-induced changes with high precision. The ability to collect real-time spectra while exposing the sample to hydrogen enabled the detection of intermediates that would be difficult to observe with other methods.
Furthermore, by combining the APXPS experimental observations with first-principles-based microkinetic modeling and simulations, the researchers gained a comprehensive understanding of the reaction mechanisms underlying hydrogen spillover.
Spectroscopic signals of spillover
The APXPS spectra revealed real-time shifts in the tungsten oxidation states, directly visualizing the stepwise tungsten reduction process associated with hydrogen spillover. Additionally, the emergence of hydroxyl and adsorbed water signatures confirms the formation of hydrogen spillover products, highlighting the coexistence of spillover pathways that involve both protons and electrons.
Temperature- and depth-dependent APXPS, in conjunction with modeling and theoretical analysis, revealed three different temperature-dependent reaction processes, resulting from the interplay between rates of hydrogen spillover (and its reverse), H+ diffusion to the bulk, and water desorption.
A surprising finding was that the spatial placement of Pt—on top or beneath the WO3 thin film—had no effect on the occurrence of hydrogen spillover, indicating that WO3 does not initiate hydrogen activation but serves as a conduit for hydrogen migration. Overall, the results provide a comprehensive understanding of the reaction mechanisms underlying hydrogen spillover on reducible metal oxides, offering valuable insights into the control of catalytic hydrogen-related transformations.
Contact: Ethan Crumlin
Researchers: H. Li and J.R. Paudel (Berkeley Lab), M. Abdelgaid and J.R. McKone (University of Pittsburgh), N.P. Holzapfel and V. Augustyn (North Carolina State University), G. Mpourmpakis (University of Pittsburgh and National Technical University of Athens, Greece), and E.J. Crumlin (Berkeley Lab and ALS).
Funding: US Department of Energy, Office of Science, Basic Energy Sciences program (DOE BES); Center for Research Computing at the University of Pittsburgh; and National Science Foundation. Operation of the ALS is supported by DOE BES.
Publication: H. Li, M. Abdelgaid, J.R. Paudel, N.P. Holzapfel, V. Augustyn, J.R. McKone, G. Mpourmpakis, and E.J. Crumlin, “Operando unveiling of hydrogen spillover mechanisms on tungsten oxide surfaces,” J. Am. Chem. Soc. 147, 6472 (2025), doi:10.1021/jacs.4c13711.
ALS SCIENCE HIGHLIGHT #520