Gold-based alloys have emerged as promising catalysts with high product selectivity: that is, they can be “tuned” to yield specific chemicals of interest. Yet, little is known about the physical tuning mechanism—the dynamic restructuring these alloys undergo as their catalytic properties are activated. To better understand these transformations, researchers have structurally and chemically “visualized” the surface of a silver–gold alloy as it reorganizes itself during the process of catalytic activation. The methodology, which includes ambient-pressure x-ray photoelectron spectroscopy (APXPS) at the ALS, provides insights that can lead to improved catalysts for energy-intensive industrial applications, thereby increasing efficiency and reducing waste.
Nanoporous gold (npAu) is a dilute alloy of 3% silver and 97% gold. Nanoscale pores and ligaments (connecting strips) provide ample surface area for catalytic reactions. As a highly crystalline alloy requiring no underlying support material, it is not complicated by lattice mismatches, intermetallic bonding, or metal–support interactions. Thus, npAu presents a straightfoward platform for investigating the step-by-step transformation of a bimetallic alloy into a working catalyst. Activation is achieved by first preconditioning the npAu surface by flowing ozone gas (O3) over it, followed by the reactive mixture of methanol (CH3OH) and oxygen (O2) at a working temperature of 150 °C. The activated npAu is an extremely robust and selective catalyst for oxygen-assisted “coupling reactions” that join simple hydrocarbon fragments into more complex end products (e.g., alcohols, esters).
To explore the physical origins of npAu activation and selectivity, the researchers used three techniques: APXPS, transmission electron microscopy (TEM), and temporal analysis of products (TAP). In the TAP experiments, the researchers exposed ozone-preconditioned npAu to repeated short pulses of reactant gases (CO and CH3OH) and tracked the product outflow over time. They observed an abrupt transition between the production of CO2 (during an initial combustion phase) and the selective oxidation of CH3OH to produce methyl formate (HCOOCH3—the simplest form of alcohol) and formaldehyde (HCHO—the simplest form of ester). The results indicated that there are two chemically distinct oxygen species on the surface: one responsible for the initial combustion and the other for the selective oxidation of CH3OH. TEM experiments on ozone-preconditioned npAu revealed a thin layer of amorphous oxide on the surface. Environmental TEM experiments (TEM in the presence of gases) showed that exposure to CO and then CH3OH resulted in the removal of the amorphous oxide layer and the growth of highly crystalline metallic nanoparticles.
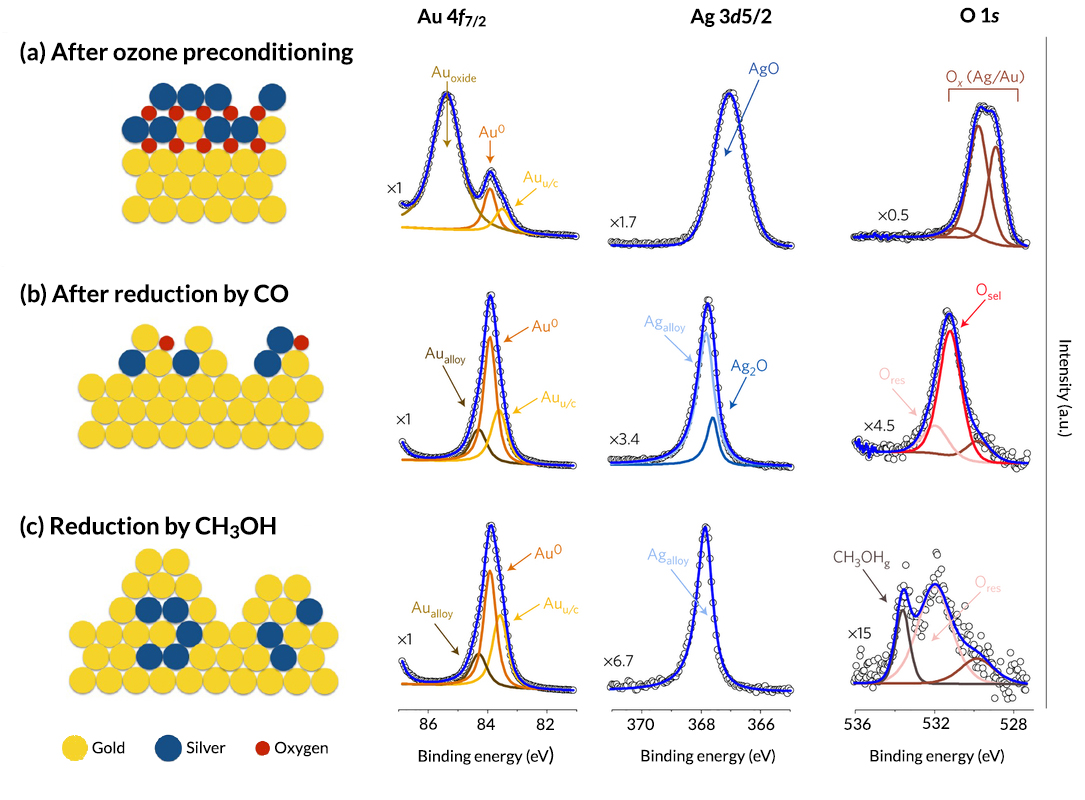
The researchers then performed APXPS studies at ALS Beamline 11.0.2. According to the data, ozone preconditioning concentrates silver near the surface as silver oxide (AgO). Thin films of gold oxide are also formed. These metallic oxides are identified as the oxygen species responsible for the initial combustion phase. A second form of oxygen (Osel), with a binding energy of 531.5 eV, is identified as the species that leads to selective oxidation of CH3OH. It appears in conjunction with the CO reduction step and persists until reduction by CH3OH, after which only a small amount of residual (subsurface) oxygen remains (Ores). In addition, a steady increase in undercoordinated gold sites (Auu/c) is attributed to the formation and growth of small crystalline particles on the npAu surface, as was observed in the TEM results.
In summary, a series of complementary studies has demonstrated that preconditioning with ozone creates a silver-rich oxide layer on the npAu surface. Removal of this reactive oxygen species by CO leads to a restructuring that reveals the distinct oxygen species that reacts with CH3OH to form selective oxidation products. The work clearly demonstrates that the behavior of alloy catalysts can be tuned using carefully constructed preconditioning methods, and that a fuller understanding of the dynamic changes that occur during preconditioning is necessary to unlock the full potential of bimetallic catalytic materials.
Contact: Cynthia Friend
Research conducted by: B. Zugic, L. Wang, R.J. Madix, and C.M. Friend (Harvard University); C. Heine, B.A.J. Lechner, and M. Salmeron (Berkeley Lab); D.N. Zakharov and E.A. Stach (Brookhaven National Laboratory); and J. Biener (Lawrence Livermore National Laboratory).
Research funding: U.S. Department of Energy, Office of Science, Basic Energy Sciences Program (DOE BES). Operation of the ALS is supported by DOE BES.
Publication about this research: B. Zugic, L. Wang, C. Heine, D.N. Zakharov, B.A.J. Lechner, E.A. Stach, J. Biener, M. Salmeron, R.J. Madix, and C.M. Friend, “Dynamic restructuring drives catalytic activity on nanoporous gold–silver alloy catalysts,” Nat. Mater. 16, 558 (2017). doi:10.1038/nmat4824
ALS SCIENCE HIGHLIGHT #355