High-energy lithium-ion batteries can provide both high capacity and high voltage, both of which are important in electric vehicles for greater range and faster acceleration. Lithium-rich transition-metal oxides, which have higher lithium content than materials currently used commercially, are promising candidates for battery cathode materials, capable of boosting battery performance by delivering high capacities (about 300 mAh/g) and high operating voltages (over 4.5 V).
Despite decades of intensive effort, commercial adoption of these materials has been hindered by the appearance of irreversible chemical activity—largely believed to involve oxygen—during high-voltage operation, leading to detrimental effects. It remains a critical task to understand the factors that affect such oxygen activity, to better guide material development.
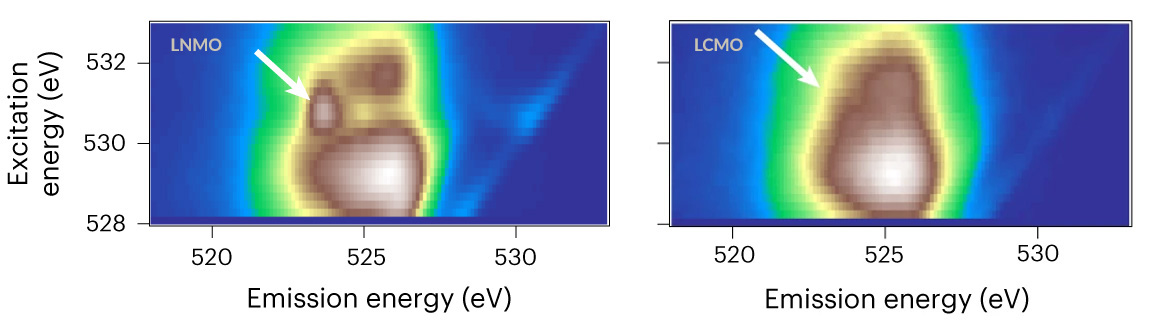
Toward this end, an international team of scientists used resonant inelastic x-ray scattering (RIXS) at Beamline 8.0.1 of the Advanced Light Source (ALS) to study two lithium-rich cathode systems featuring different transition metals—one with nickel, the other with cobalt.
“The goal was to use soft x-ray RIXS to reveal the different chemical reactions occurring in the two systems when the batteries are operated at high voltage,” said Zengqing Zhuo, a postdoctoral researcher at the ALS who was primarily responsible for performing the RIXS experiments. Additional techniques used in this study included powder x-ray diffraction, transmission electron microscopy, x-ray absorption spectroscopy.
The researchers found that cobalt is more effective than nickel in mediating the kinetics of a charge-transfer process involving oxygen oxidation, and it favors more transition-metal migration (i.e., structural reorganization). This results in less cationic redox but more oxygen redox, leading to increased oxygen release, poorer cycling performance, and more-severe voltage decay.
“By leveraging RIXS spectroscopy data, we have successfully untangled the distinct contributions of nickel and cobalt in lithium-rich battery cathodes,” said Wanli Yang, a senior scientist at the ALS. “The results offer valuable insights into the design of high-capacity cathodes and points the way to a compositional approach to optimizing lithium-rich oxide cathodes.”
B. Li, Z. Zhuo, L. Zhang, A. Iadecola, X. Gao, J. Guo, W. Yang, A.V. Morozov, A.M. Abakumov, and J.-M. Tarascon, “Decoupling the roles of Ni and Co in anionic redox activity of Li-rich NMC cathodes,” Nat. Mater. 22, 1370 (2023), doi:10.1038/s41563-023-01679-x.