Although lithium-ion batteries currently drive the electronic world, sodium-ion batteries are gaining ground. A big plus is that sodium is more earth-abundant than lithium, which lowers costs and eases environmental and supply-chain concerns. As a result, enormous research efforts are underway to improve the energy density and cycling longevity of sodium-ion batteries.
One promising strategy to enhance energy density is to exploit additional sources of a key chemical reaction—oxidation reduction (redox)—in the battery’s cathode. In cathodes made of layered transition-metal oxides (the sodium ions move in and out from between the layers), it turns out that the oxygen component in the layers unexpectedly contributes to redox activity. Unfortunately, oxygen redox is frequently accompanied by irreversible oxygen behavior, resulting in both voltage and capacity decays.
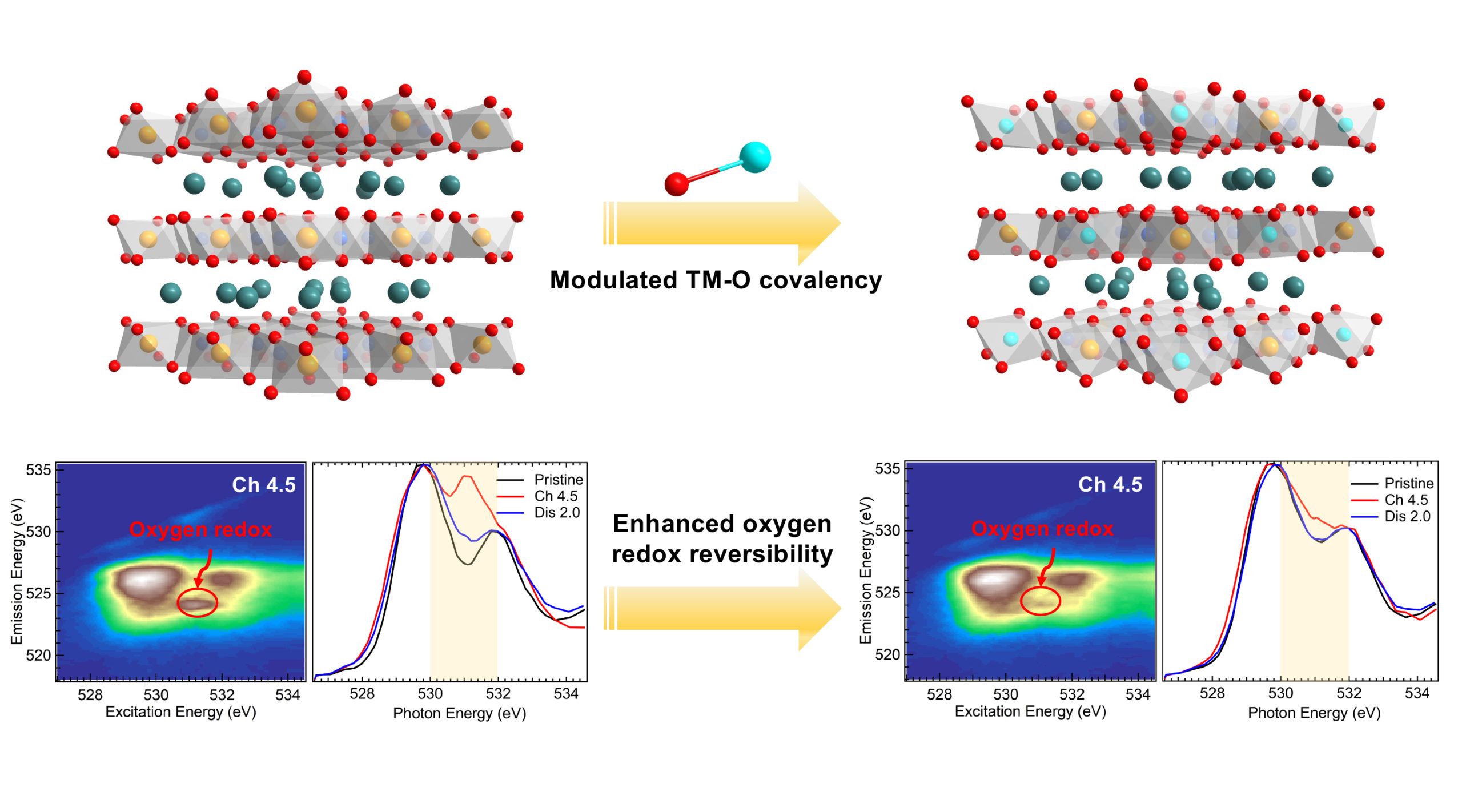
To enhance oxygen redox reversibility, researchers doped a sodium-ion cathode material (Na0.6Mg0.3Mn0.7O2, or NMMO) with copper ions (Na0.6Mg0.15Mn0.7Cu0.15O2, or NMMCO). To understand how this affects the oxygen redox activity, they used a technique developed at Beamline 8.0.1 of the Advanced Light Source (ALS), called mapping of resonant inelastic x-ray scattering (mRIXS), to measure the effect of the copper doping at different states of charge.
“The mRIXS technique is one of the most powerful characterization tools available for detecting oxygen redox activities in battery electrodes,” said Liang Zhang, a professor at Soochow University and co-corresponding author of the published work. “With mRIXS, it’s also possible to quantify the reversibility of the oxygen redox reaction, which could greatly help us understand the oxygen evolution process during electrochemical cycling.”
The mRXIS results, combined with first-principles calculations and electrochemical characterizations, revealed that the copper doping results in a more rigid oxygen lattice, which leads to less distortion upon sodium-ion extraction and insertion. The oxygen redox reversibility improved from 73% for NMMO to 95% for NMMCO. The NMMCO also demonstrated less voltage fade and improved capacity retention (95.8%) after 200 cycles at a high current rate.
The work emphasizes the importance of reliable characterization techniques that distinguish between different sources of oxygen activity in order to develop high-performance cathodes through rational design rules.
C. Cheng, C. Chen, S. Chu, T. Yan, H. Hu, X. Xia, X. Feng, J.-H. Guo, D. Sun, J. Wu, S. Guo, and L. Zhang, “Enhancing the Reversibility of Lattice Oxygen Redox Through Modulated Transition Metal–Oxygen Covalency for Layered Battery Electrodes,” Adv. Mater. 34, 2201152 (2022), doi:10.1002/adma.202201152.