SCIENTIFIC ACHIEVEMENT
As revealed by x-ray microscopy at the Advanced Light Source (ALS), researchers controlled the mixing of electron-donating and -accepting constituents of an organic photovoltaic (OPV) material made using a process that replaces toxic solvents with water.
SIGNIFICANCE AND IMPACT
With efficiencies comparable to less eco-friendly OPVs, this material shows promise for many advanced device and building applications.
A “greener” path to solar power
Organic (carbon-based) photovoltaics (OPVs) offer many advantages over traditional silicon solar panels. OPVs can be manufactured inexpensively at industrial scale using solution-based deposition techniques onto rolls of flexible substrates. The end result is a thin, lightweight, and bendable photoactive material that can take on different colors and varying degrees of transparency. While less efficient in converting light into electricity than classic silicon solar cells, OPVs can potentially be used in more ways, including in tensile-fabric architectural structures, rollable solar panels for disaster relief operations, and printed bioelectronic devices.
However, fabrication of photoactive OPV films typically involves solutions of organic semiconductors dissolved in toxic solvents. Although a cleaner way is possible by synthesizing organic semiconductor nanoparticles dispersed in water, it has so far resulted in lower power-conversion efficiencies. In this work, an international team of researchers discovered a way to optimize the makeup of the nanoparticles, which allowed water-processed OPV films to reach higher efficiencies.
A more intimate morphology
Optimizing the mixing between electron-donating and electron-accepting phases in the photoactive layer is key to increasing solar-cell efficiency. Thorough intermixing encourages the separation of electron–hole pairs created by the absorption of light. It also facilitates the efficient transport of the charges to produce an electric current. Many organic molecules—polymers and fullerenes, for example—offer great potential as donor and acceptor phases, respectively.
However, studies have shown that, because of high surface energy (which tends to minimize surface area), fullerene acceptor phases will migrate to nanoparticle cores, while the polymer donor phases form an outer shell. Such core–shell arrangements (morphologies) can trap charges in the core, degrading device performance. Thermal annealing can help by creating connections between nanoparticle cores, but a more intimate morphology is still needed to increase efficiency.
A closer look at non-fullerene acceptors
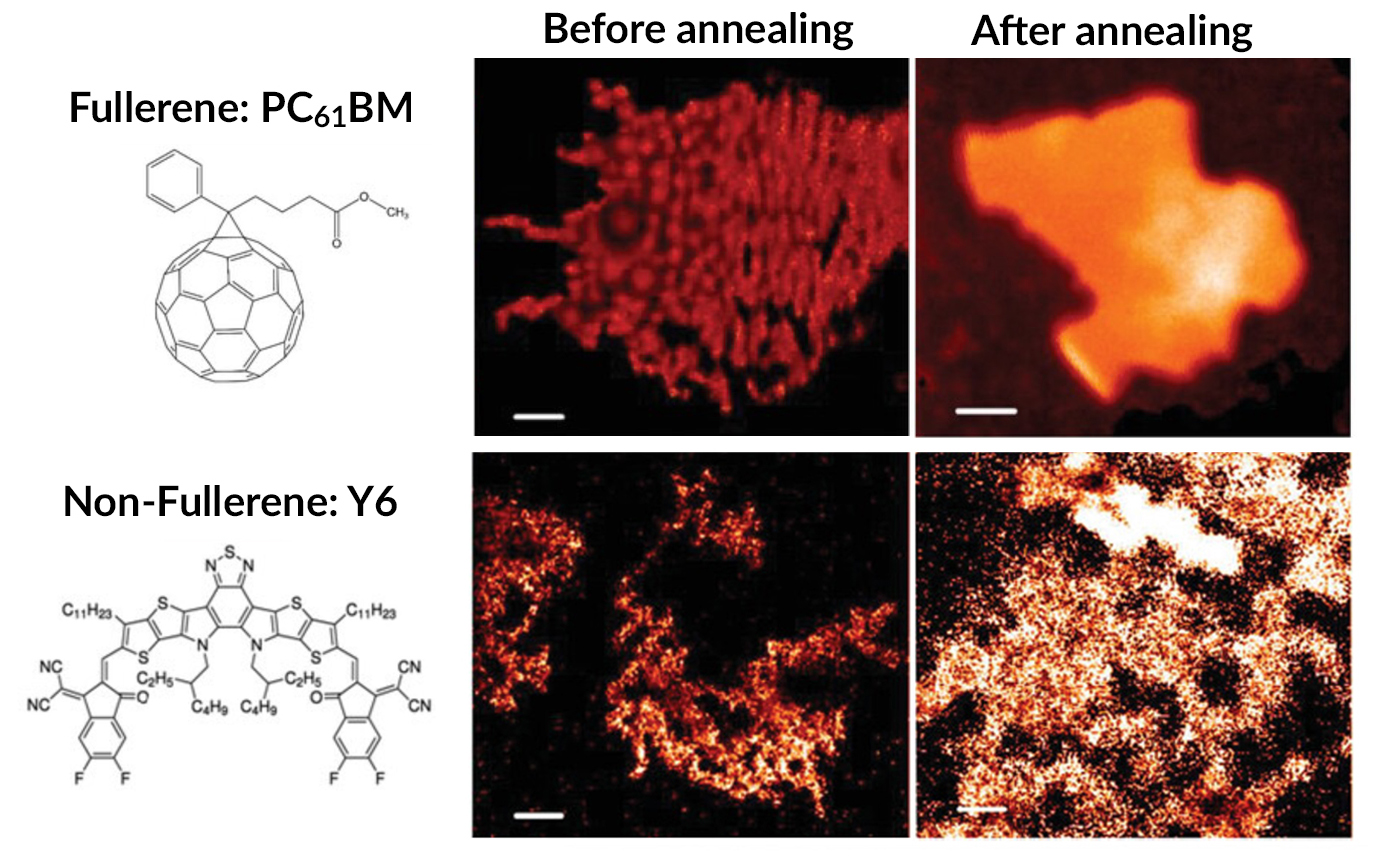
In this study, the researchers prepared two types of OPV nanoparticles: one with fullerene acceptors (PC61BM) and the other with non-fullerene acceptors (Y6). The latter was chosen to have a surface energy that more closely matched that of the polymer donor phase (PTQ10), reducing the interfacial energy difference between them.
Scanning transmission x-ray microscopy (STXM) at ALS Beamline 5.3.2.2 was used to compare the resulting nanoparticle morphologies. The beamline covers the carbon K edge with high energy resolution, enabling the researchers to distinguish between organic semiconductors with similar chemical structures at the nanoscale.
Narrowing the efficiency gap
The STXM data confirmed that a large surface-energy difference between donor and acceptor materials leads to a core–shell morphology. The smaller energy difference in the non-fullerene case resulted in a well-mixed morphology, which improves performance. Moreover, this intermixing persisted even after heating, which removes surfactants used during nanoparticle synthesis and fuses crystalline domains, both of which improve performance.
The power-conversion efficiencies measured for photoactive films produced using these two types of nanoparticles dispersed in water yielded about 1% for the fullerene material and 9.98% for the non-fullerene material. To the researchers’ knowledge, the latter value is the highest performance achieved for water-processed OPV devices without additives. It significantly closes the gap with the 11.2% efficiency of films produced using a chlorine-based solvent, a promising development for the eco-friendly processing and fabrication of organic photovoltaics.
Contact: Natalie Holmes
Researchers: H. Laval, R. Szymanski, and G. Wantz (University of Bordeaux, France); A. Holmes, C. Lartigau-Dagron, and A. Bousquet (University of Pau and the Pays de l’Adour [UPPA], France); M.A. Marcus (ALS); B. Watts (Paul Scherrer Institute, Switzerland); G. Bonfante, K. Hirakawa, F. Awai, and T. Kubo (University of Tokyo, Japan); M. Schmutz (University of Strasbourg, France); E. Deniau (UPPA and University of Le Mans, France); X. Xu (University of Technology Sydney, Australia); J.M. Cairney and N.P. Holmes (University of Sydney, Australia); and S. Chambon (University of Bordeaux and University of Tokyo).
Funding: French National Research Agency (ANR); French National Center for Scientific Research (CNRS); Research Center for Advanced Science and Technology (RCAST), University of Tokyo; Japan Society for the Promotion of Science (JSPS); University of Bordeaux; Energy and Environment Solutions (E2S), UPPA; International Higher Institute for Research Training (ISIFoR), France; German Federal Ministry of Education and Research (BMBF); and Australian Nuclear Science and Technology Organisation. Operation of the ALS is supported by the US Department of Energy, Office of Science, Basic Energy Sciences program.
Publication: H. Laval, A. Holmes, M.A. Marcus, B. Watts, G. Bonfante, M. Schmutz, E. Deniau, R. Szymanski, C. Lartigau-Dagron, X. Xu, J.M. Cairney, K. Hirakawa, F. Awai, T. Kubo, G. Wantz, A. Bousquet, N.P. Holmes, and S. Chambon, “Toward High Efficiency Water Processed Organic Photovoltaics: Controlling the Nanoparticle Morphology with Surface Energies,” Adv. Energy Mater. 13, 202300249 (2023); doi:10.1002/aenm.202300249.
ALS SCIENCE HIGHLIGHT #490