The increasing popularity of hybrid and electric vehicles is driving a surge in global lithium demand, necessitating a robust and reliable supply. Currently, most lithium-ion battery cathodes are synthesized from lithium carbonate (Li2CO3) or lithium hydroxide (LiOH), both of which can be obtained by processing brines or hard rocks. While brine extraction is common, the processing of hard rocks—particularly spodumene (LiAlSi2O6)—is faster and typically yields a higher recovery rate.
Lithium extraction from spodumene involves first transforming α-spodumene (the naturally occurring form) into β-spodumene (a high-temperature polymorph) through calcination (heating above 1000 °C). Then, the mineral is acid roasted (heated in strong acid) before undergoing neutralization to precipitate Li2CO3.
“These processes are both energy intensive and costly, posing significant sustainability and economic challenges,” said Shilong Wang, a graduate student in the Materials Science and Engineering Department at UC Berkeley and first author of a study that presents an alternative method. “To develop an energy-saving, acid-free route, we explored direct lithium extraction from α-spodumene through solid-state reactions.”
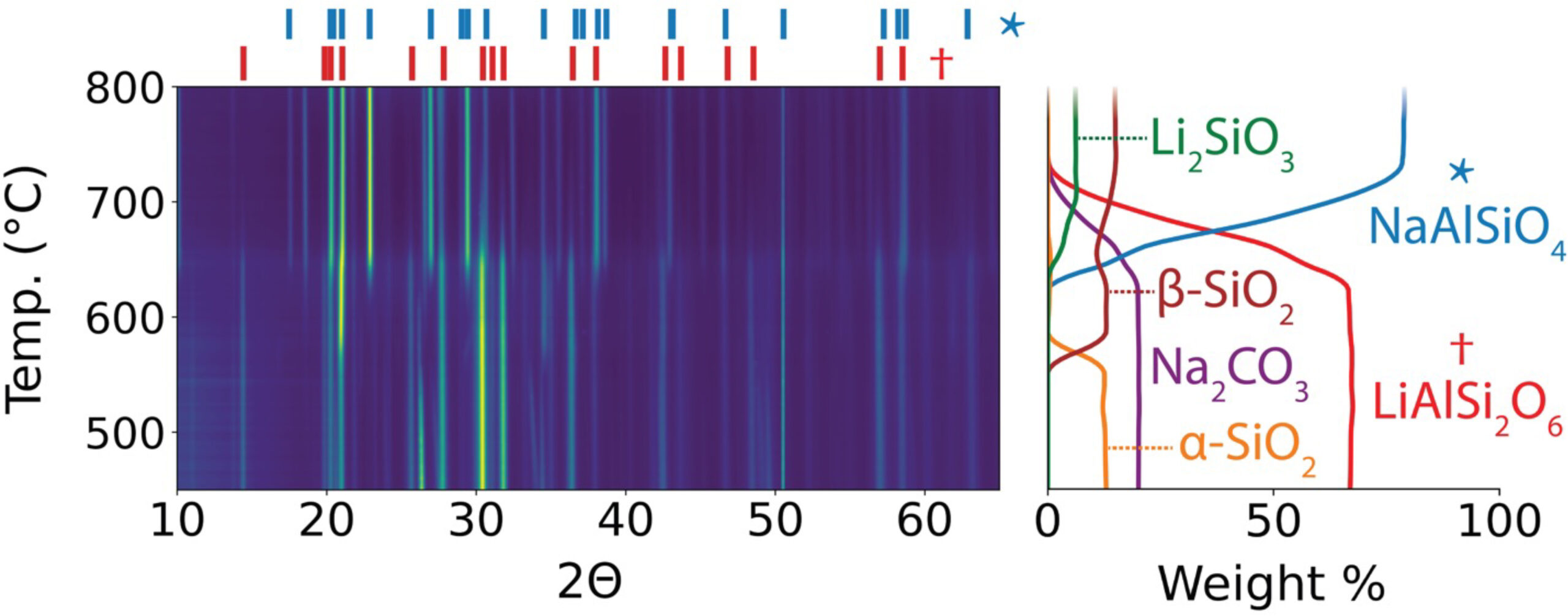
In this work, the researchers first introduced sodium carbonate (Na2CO3) directly to α-spodumene. In situ x-ray diffraction (XRD) experiments at Advanced Light Source (ALS) Beamline 12.2.2 enabled real-time monitoring of the reaction and its products. The results showed the formation of Li2CO3, but in quantities that suggest only 76% of the lithium was extracted. The limited yield was attributed to the competing formation of lithium metasilicate (Li2SiO3), which traps some Li+ in an inactive phase.
To avoid this byproduct, the researchers identified aluminum oxide (Al2O3) as an additive that would trap silicate anions and provide increased thermodynamic driving force to form Li2CO3. The reaction with both Na2CO3 and Al2O3 was tested experimentally, and this time it was found that 90.4% of the lithium was successfully extracted from spodumene in the form of Li2CO3, which could be isolated using a low-cost washing procedure without acid.
“This advancement is significant because it enables a more efficient and less energy-intensive extraction process, ultimately making the production of lithium precursors more sustainable,” said Wang. “These improvements could eventually reduce battery costs for electric cars and renewable energy systems.”
S. Wang, N. Szymanski, Y. Fei, W. Dong, J.N. Christensen, Y. Zeng, M. Whittaker, and G. Ceder, “Direct lithium extraction from β-spodumene through solid-state reactions for sustainable Li2CO3 production,” Inorg. Chem. 63, 13576 (2024), doi:10.1021/acs.inorgchem.4c01722.