SCIENTIFIC ACHIEVEMENT
Researchers studied a novel approach to ammonia synthesis by measuring the surface hydroxylation of magnetite nanoparticle catalysts under ambient, in situ conditions at the Advanced Light Source (ALS).
SIGNIFICANCE AND IMPACT
These experimental and modeling results pave the way for more efficient and economical ammonia production.

Toward a more efficient path to ammonia
Agriculture depends on ammonia, a critical ingredient in most fertilizers. Chemical manufacturing also relies on ammonia for various processes, from the production of pharmaceuticals to cleaning agents.
Outside of lightning strikes, decomposition, and specialized bacteria, ammonia does not readily form in nature. The Haber–Bosch process is the main industrial process for ammonia production. It typically uses hydrogen from fossil sources, requires very high temperatures and pressures, and is a large source of carbon dioxide emission.
Recent experiments have suggested that magnetite (Fe3O4) nanoparticles in the presence of microdroplets of water and nitrogen gas under near-ambient conditions offer an alternate process to create ammonia. Until recently the mechanism behind this process remained unclear. This new study provides the first direct spectroscopic evidence of surface hydroxylation (water splitting) and nitrogen adsorption in the production of ammonia.
Beamline data agrees with theoretical predictions
Researchers from two divisions within Lawrence Berkeley National Laboratory (Berkeley Lab)—the ALS and the Chemical Sciences Division—collaborated to combine ambient-pressure x-ray photoelectron spectroscopy (APXPS) data from the ALS with ab initio computational methods to clarify the role of hydroxylation and active surface sites on magnetite nanoparticles that enable nitrogen reduction.
The team used APXPS at two ALS beamlines. Beamline 9.3.2, which produces low-energy, soft x-rays, exposed magnetite nanoparticles placed on gold foil to varying pressures of water vapor and nitrogen gas, as well as different temperatures. Under these experimental conditions, the team aimed to understand hydroxyl formation on the nanoparticle surface by measuring the core level spectra of iron, nitrogen, oxygen, and carbon. The team used Beamline 9.3.1, which offers medium-energy, tender x-rays, to expose the samples to higher photon excitation and thus allowed to increase the water vapor and nitrogen gas pressures during the experiments.
In parallel, the team conducted advanced quantum chemistry-based simulations that clarified how water molecules split and create special surface sites on the magnetite nanoparticles. The sites provide locations where the nitrogen reduction takes place. Ab initio molecular dynamics and free energy calculations also confirmed that hydroxylated magnetite surfaces lower energetic barriers for nitrogen reduction, which enables diverse binding geometries and proton-coupled electron transfer mechanisms.
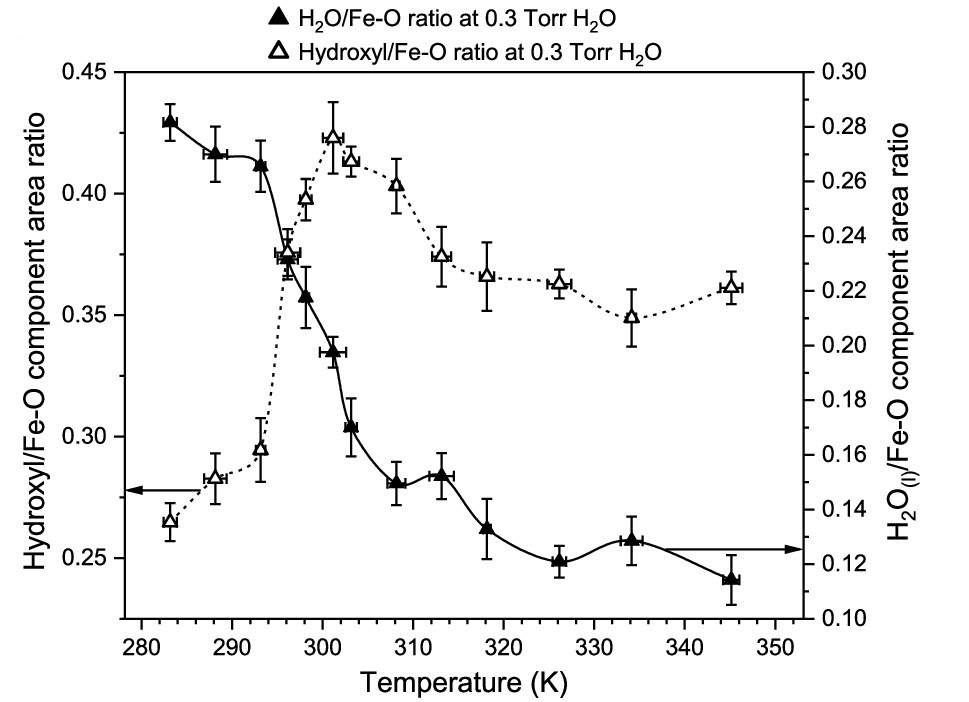
A mechanism explained
This is the first study to provide an integrated experimental–theoretical foundation for understanding the atomic-scale mechanism behind ammonia synthesis at water–magnetite interfaces that drive nitrogen fixation under ambient conditions, a longstanding goal of sustainable chemistry. This new approach shifts the paradigm from viewing nitrogen binding as a rate-limiting step that hinders ammonia production to recognizing that water activation can enable and enhance catalytic activity. This insight can guide future efforts for ammonia synthesis with low energy input and minimal carbon emissions.

Contacts: Monika Blum and Teresa Head-Gordon
Researchers: S.K. Chandy and T. Head-Gordon (University of California, Berkeley and Chemical Sciences Division, Berkeley Lab); M. Blum and A. Siebert (Chemical Sciences Division and ALS, Berkeley Lab); N.Z. Rustad, I.N. Zakaria, and S. Devlin (ALS); M. Lopez Luna (Chemical Sciences Division, Berkeley Lab); and W. Li (University of California, San Diego)
Funding: Condensed Phase and Interfacial Molecular Science (CPIMS) program funded by the Chemical Sciences, Geoscience, and Biosciences (CSGB) division of the US Department of Energy (DOE) Office of Basic Energy Sciences (BES); ALS Doctoral Fellowship in Residence program; and National Defense Science and Engineering Graduate Fellowship program funded by the US Department of Defense. This project used the resources of the ALS and National Energy Research Scientific Computing Center, which are supported by DOE BES and the DOE Advanced Scientific Research Computing program, respectively, as well as Berkeley Lab’s Lawrencium computational cluster.
Publication: S. K. Chandy, M. Lopez Luna, N.Z. Rustad, I.N. Zakaria, A. Siebert, S. Devlin, W. Li, M. Blum, and T. Head-Gordon, “Ammonia synthesis under ambient conditions: Insights into water−nitrogen−magnetite interfaces,” Journal of the American Chemical Society 147, 24538-24547 (2025). doi:10.1021/jacs.5c04852
ALS SCIENCE HIGHLIGHT #527