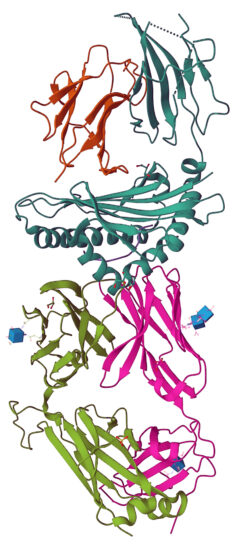
A protein structure obtained at Beamline 2.0.1 (“Gemini”) at the Advanced Light Source (ALS) has recently been published in the literature and deposited into the Protein Data Bank (PDB)—two significant firsts for this beamline. The structure helped provide new insights into the molecular mechanisms involved in triggering certain inflammatory diseases.
The study was done by K. Christopher Garcia’s group at Stanford University and the Howard Hughes Medical Institute (HHMI), in collaboration with groups at Oxford and Washington University. It was led primarily by Xinbo Yang, a postdoc in Garcia’s group, who worked with research specialist Kevin Jude to conduct the protein structure studies.
“Gemini is our first microfocus beamline, designed to target really small crystals,” said beamline scientist Marc Allaire, a staff scientist with the Berkeley Center for Structural Biology (BCSB). “There is a rule in crystallography that if you want a better signal from your crystal, the beam needs to be about the size of the crystal. The beam size that we’re getting right now is smaller than 20 microns, versus 50 to 200 from other beamlines that we have on the floor.”

Initial funding for the beamline came from a proposal that Simon Morton (now a semi-retired ALS staff scientist) and Corie Ralston (currently a facility director at the Molecular Foundry) presented to HHMI. Berkeley Lab provided funding to build Leda, the in-vacuum undulator source. Industrial and academic partners contributed additional funds, and NIH paid for the detector and goniometer.
“It’s just a beautiful system, where we can rapidly get answers and the beam is so bright we can solve structures with very poor crystals,” said Garcia. “It is an amazing resource that is key to our studies on human disease and therapeutic development. It certainly played a big role in our ability to make a major advance in a 50-year-old problem in human immunology and autoimmune disease.”