Proteins are macromolecular machines, and like all machines, they accumulate both mechanical and chemical damage. However, because the bonds that hold these machines together have a life span of over 300 years, they do not spontaneously degrade. To prevent cells from becoming clogged with aged and defective proteins, the ubiquitin–proteasome system has evolved. In this system, a small marker protein, ubiquitin, is attached to a target protein. Once it has been “ubiquitinated” multiple times, the target protein is digested into its component amino acids by the proteasome. A critical component of this system is the E3 ligase, which recognizes proteins to be cleared and tags them with ubiquitin.
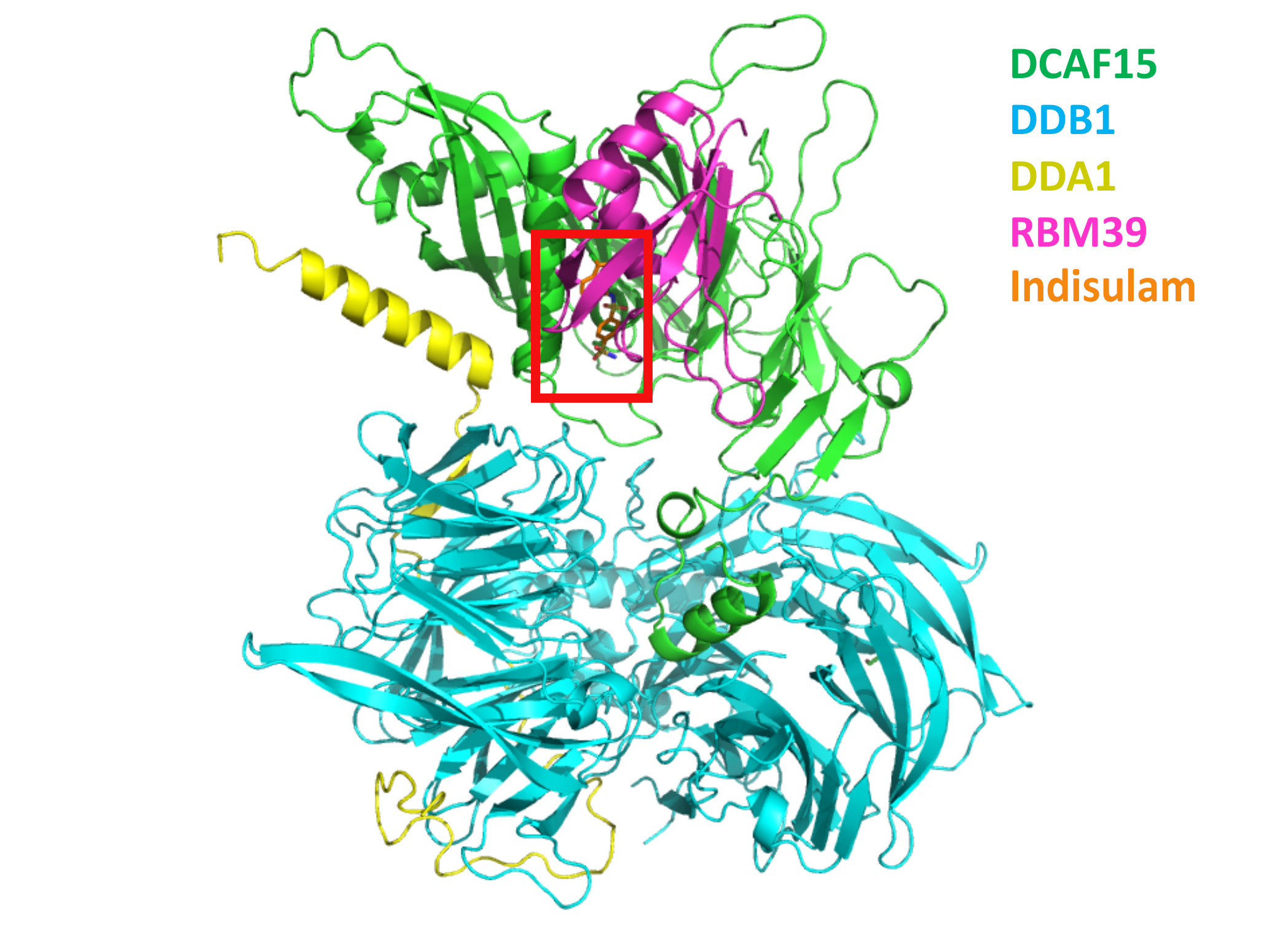
Indisulam is an anticancer drug that helps aim this protein-targeting system at RBM39, a protein responsible for a key step in cell proliferation, mRNA splicing. Researchers from Novartis wanted to explore how indisulam promotes interaction between RBM39 and the E3 ligase, DCAF15, leading to RBM39 degradation and inhibition of cancer-cell growth. The study used both protein crystallography at ALS Beamline 5.0.2 (part of the Berkeley Center for Structural Biology) and cryogenic electron microscopy.
The structures show a tight interaction between indisulam and DCAF15, leading to the formation of a four-protein complex, DCAF15-DDB1-DDA1-RBM39, where DDB1 and DDA1 are bridging and stabilizing proteins. Indisulam mediates the interaction between DCAF15 and the central helix of an RNA recognition motif on RBM39. This detailed understanding of indisulam’s mechanism of action is the first step toward determining whether the DCAF15 E3 ligase can be reprogrammed by other small molecules to degrade new targets beyond RBM39.
D.E. Bussiere, L. Xie, H. Srinivas, W. Shu, A. Burke, C. Be, J. Zhao, A. Godbole, D. King, R.G. Karki, V. Hornak, F. Xu, J. Cobb, N. Carte, A.O. Frank, A. Frommlet, P. Graff, M. Knapp, A. Fazal, B. Okram, S. Jiang, P.-Y. Michellys, R. Beckwith, H. Voshol, C. Wiesmann, J.M. Solomon, and J. Paulk, “Structural basis of indisulam-mediated RBM39 recruitment to DCAF15 E3 ligase complex,” Nat. Chem. Biol. 16, 15 (2020), doi:10.1038/s41589-019-0411-6.