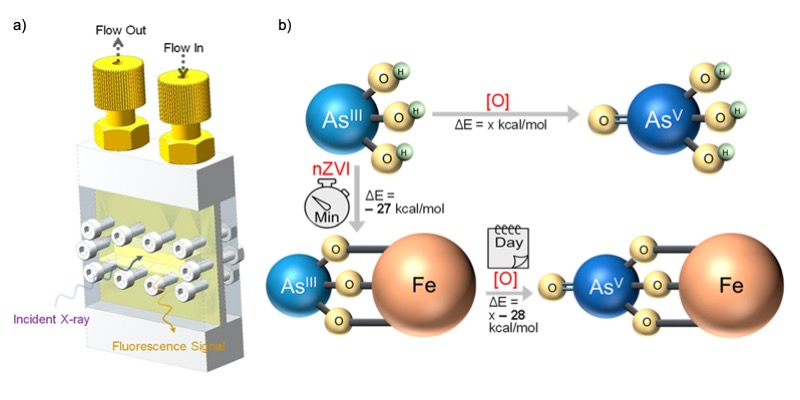
Arsenic contamination in groundwater arises from both natural causes as well as common practices in the community and threatens the environment and human health. Iron nanoparticles, which bind easily to arsenic and have high surface areas, have been recognized and used as an effective means to sequester, or neutralize, the contaminant. However, arsenic remediation strategies aren’t as robust as they could be because scientists haven’t understood the details of how arsenic and iron interact.
To reveal the mechanism and transient intermediate products in the reaction between the iron and arsenite—one of the primary forms of arsenic in natural waters—an international team of scientists used soft x-ray absorption spectroscopy (XAS) and resonant inelastic x-ray scattering (RIXS) at Advanced Light Source (ALS) Beamlines 7.3.1 and 8.0.1. The researchers designed a specialized flow cell that enabled them to perform x-ray analysis under realistic conditions as the iron nanoparticles flowed through an arsenite solution under anaerobic (oxygen-free) conditions, simulating a groundwater environment.
The combination of techniques revealed the oxidation state of the iron nanoparticles—a chemical state associated with catalytic activity—in areas less than 30 nanometers in diameter. Researchers then combined the real-time XAS data with theoretical calculations to show that the expected arsenic sequestration process is energetically favored and can be completed in a few minutes.
An even more interesting process unfolded afterward, as they continued to model and experimentally monitor the reaction. “We found that the iron nanoparticles can facilitate the oxidation of the arsenite into a less toxic, arsenate species over the course of a few days,” said Jinghua Guo, an ALS senior scientist and co-corresponding author of the study.
“The secret of the role of the iron nanoparticles is revealed for the first time,” Guo added. The new information paves the way for improved remediation strategies and a cleaner water future.
L.C. Kao, Y. Ha, W.-J. Chang, X. Feng, Y. Ye, J.-L. Chen, C.W. Pao, F. Yang, C. Zhu, W. Yang, J. Guo, and S.Y.H. Liou, “Trace Key Mechanistic Features of the Arsenite Sequestration Reaction with Nanoscale Zerovalent Iron,” J. Am. Chem. Soc. 143, 40 (2021), doi: 10.1021/jacs.1c06159.