SCIENTIFIC ACHIEVEMENT
The structure of an antibody was modified to selectively activate a specific pathway of the immune system, demonstrating its value in killing tumor cells.
SIGNIFICANCE AND IMPACT
The work provides a platform for disentangling the effects of different immune-system pathways and could lead to the design of improved immunotherapies.
The immunotherapy approach
Immunotherapy—the use of the immune system to fight disease—has made tremendous progress in the fight against cancer. Antibodies such as immunoglobulin G (IgG) can identify and attack foreign or abnormal substances, including tumor cells. But to control and amplify this response, scientists need to know more about how elements of the immune system recognize tumor cells and trigger their destruction. There are two main pathways for this: antibody-dependent mechanisms and complement-dependent mechanisms.
The antibody pathway involves coating the surfaces of tumor cells with antibodies that recruit “natural killer” (NK) cells and macrophages (a type of white blood cell) to destroy the tumor cells. The complement pathway (so named because it complements the antibody pathway) also engages NK cells and macrophages and includes a third mechanism—a cascade of events culminating in tumor-cell destruction via a membrane attack complex (MAC).

Antibodies engineered for one pathway
Because both the antibody and complement pathways are triggered by the same IgG antibodies, disentangling their effects is difficult. The lower arm of the Y-shaped antibody (the “Fc tail”) contains binding sites for molecules that initiate the antibody pathway (FcγR) as well as for molecules that trigger the complement pathway (C1q).
By making a few amino-acid substitutions, researchers were able to modify a native (“wild type”) IgG antibody so that it would bind exclusively to C1q. The resulting monoclonal antibody (mAb), denoted A801, thus decouples the complement pathway from the antibody pathway. Such mAbs with absolute C1q-binding selectivity provide a much-needed platform for studying complement-dependendent cell-killing mechanisms, a topic that has suffered from a lack of experimental tools with sufficient specificity.
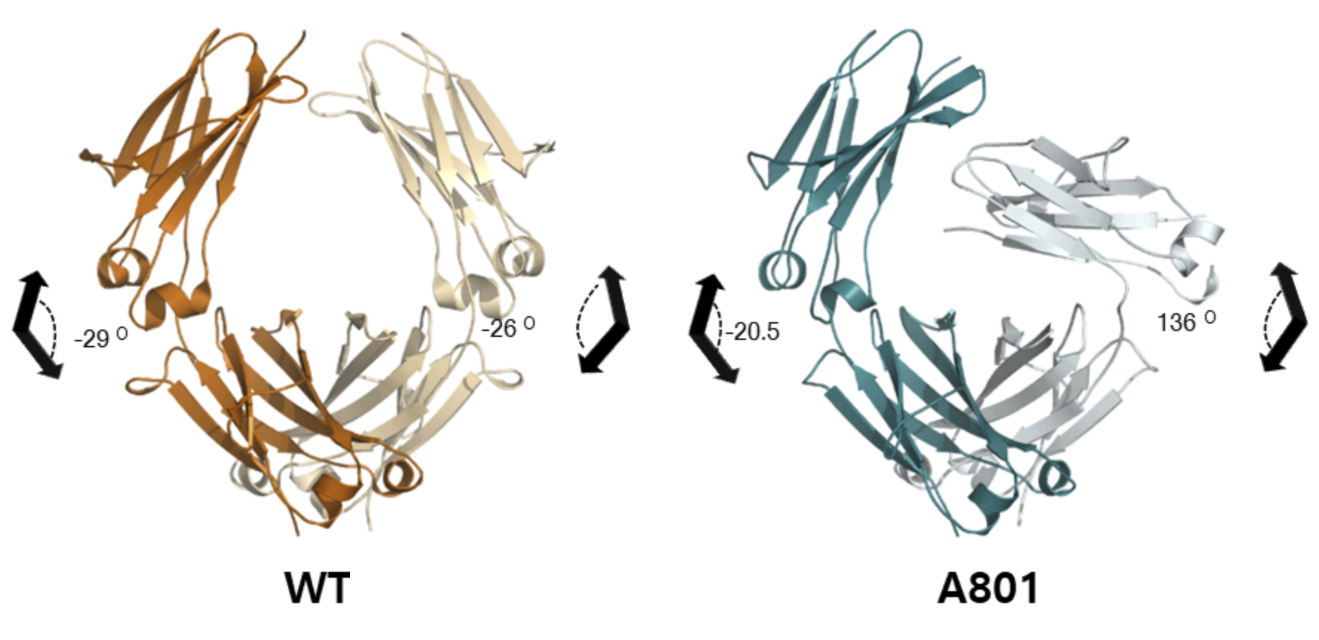
Crystallography reveals a twisted tail
To understand the molecular basis of the unique selectivity of the A801 variant, the researchers solved the crystal structure of the Fc tail and compared it to the unmodified wild-type version. The protein crystallography data were collected at ALS Beamline 5.0.3 (part of the Berkeley Center for Structural Biology) and Beamline 23-IDD at the Advanced Photon Source.
Although the IgG Fc tail is a homodimer (i.e., it’s composed of two identical subunits), the A801 variant displayed an extreme twist in the angle between the planes of the second subunit. The details of this asymmetry in the structure—which include changes in chain flexibility, variations in electrostatic interactions, and loss of access to binding sites—provided a structural explanation for enhanced C1q binding and lack of FcγR recognition.
Evaluation of anti-tumor effects
The researchers applied the engineered Fc tails to well-documented therapeutic antibodies (rituximab) to evaluate their ability to kill tumor cells in vitro as well as in mouse models. The results showed that complement-dependent mechanisms very effectively cleared tumor cells with speed and efficacy comparable to those of antibody-dependent mechanisms, while circumventing some of the adverse reactions of the latter.
Collectively, the data highlight the importance of complement-dependent mechanisms in mAb function and provide an experimental approach for delineating the effects of the complement pathway in immunotherapeutic treatments and studies.
Contact: Chang-Han Lee
Researchers: C.-H. Lee, W. Yan, M. Watanabe, W. Charab, J. Lee, K. Triplett, M. Donkor, O.I. Lungu, N. Marshall, T.H. Kang, H. Tanno, G. Delidakis, C. Alford, Y.J. Zhang, and G. Georgiou (Univ. of Texas at Austin); G. Romain and N. Varadarajan (Univ. of Houston); B. Todorova, O.-L. Goff, and P. Bruhns (Institut Pasteur and INSERM, France); A. Lux and F. Nimmerjahn (Univ. of Erlangen-Nürnberg, Germany); M.A. Lindorfer and R.P. Taylor (Univ. of Virginia); and B. Balbino (Institut Pasteur, INSERM, and Université Pierre et Marie Curie, France).
Funding: Clayton Foundation, Institut Pasteur, INSERM, European Research Council, Pasteur–Paris University International PhD Program, Cancer Prevention Research Training Program, American Cancer Society, Uehara Memorial Foundation, Japan Society for the Promotion of Science, Deutsche Forschungsgemeinschaft, National Institutes of Health, and Welch Foundation. Operation of the ALS is supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences Program.
Publication: C.-H. Lee, G. Romain, W. Yan, M. Watanabe, W. Charab, B. Todorova, J. Lee, K. Triplett, M. Donkor, O.I. Lungu, A. Lux, N. Marshall, M.A. Lindorfer, O.-L. Goff, B. Balbino, T.H. Kang, H. Tanno, G. Delidakis, C. Alford, R.P. Taylor, F. Nimmerjahn, N. Varadarajan, P. Bruhns, Y.J. Zhang, and G. Georgiou, “IgG Fc domains that bind C1q but not effector Fcγ receptors delineate the importance of complement-mediated effector functions,” Nat. Immunol. 18, 889 (2017), doi:10.1038/ni.3770.
ALS SCIENCE HIGHLIGHT #367