SCIENTIFIC ACHIEVEMENT
“Molecular anvils” (diamondoids) were used to trigger chemical reactions using pressure, yielding products that differ from those produced in conventionally driven reactions with the same reactants.
SIGNIFICANCE AND IMPACT
The discovery opens up new possibilities for the high-specificity synthesis of valuable but challenging molecules in an environmentally friendly process.
Diamondoid serendipity
For about a decade, researchers from the Stanford Institute for Materials and Energy Sciences at Stanford University have pursued a research program involving diamondoids—nanosized diamonds made up of as few as 10 carbon atoms. The diamondoids are extracted from petroleum fluids, sorted by size and shape, and put to work in ingenious ways, such as directing the self-assembly of nanowires made of copper and sulfur.
Initially, the researchers had thought that this nanowire material could be a good superconductor, so they performed experiments and calculations to test their ideas. What they found, however, was completely unexpected: the diamondoid–copper–sulfur building blocks they had synthesized did not make a superconductor, but they could be used to trigger chemical reactions with pressure, as opposed to heat or light.
Forging molecular anvils
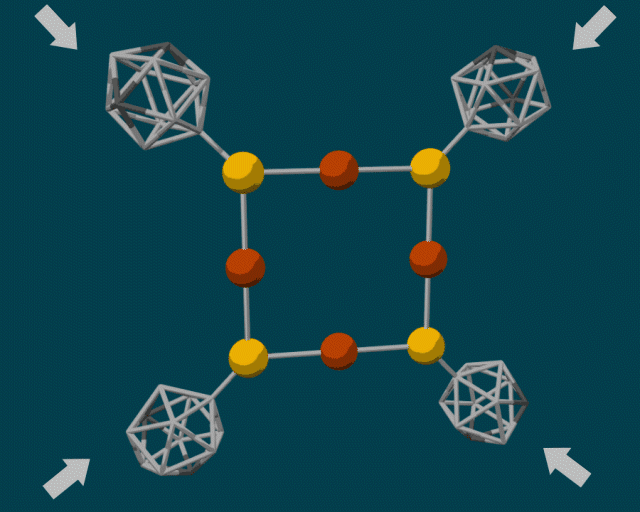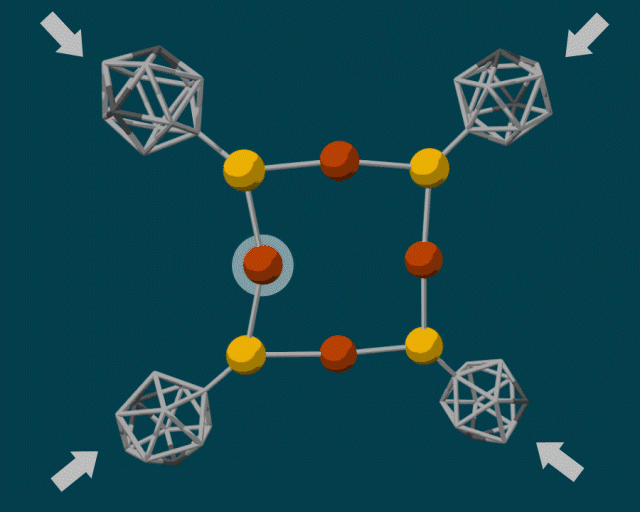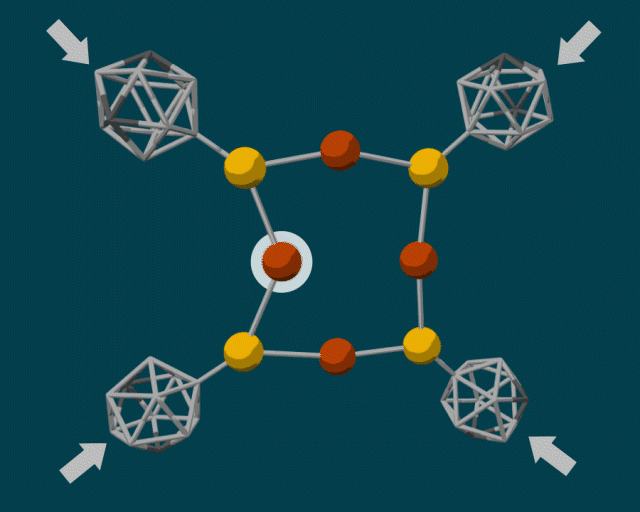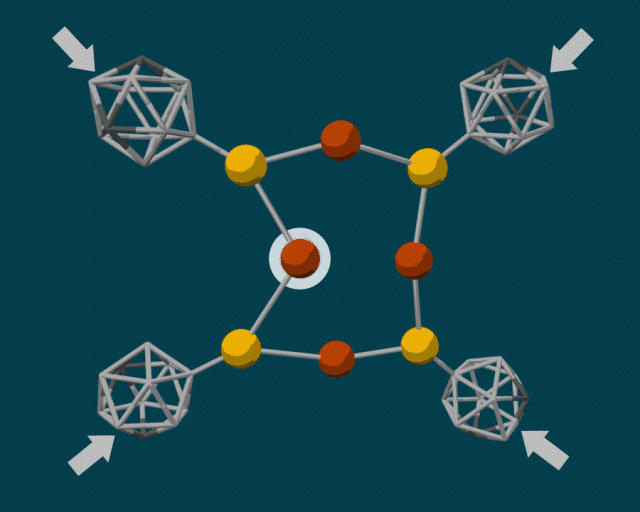
To make the diamondoid building blocks, the researchers used a guided self-assembly process. Because diamondoids are attracted to each other, they tend to form clumps, fitting together in certain ways based on their size and shape. The end result is a core–shell structure with the “soft parts” (e.g., copper and sulfur atoms) in the center and the “hard parts” (diamondoids) on the outside.
In some cases, the diamondoids are closely packed; in others, they are sparsely packed. These two configurations can produce very different reactions when external force is applied. A closely packed outer shell produces steric blockage, protecting the center from external pressure. Sparsely packed diamondoids, however, can move relative to each other—like molecular anvils—deforming the soft parts in the center and driving reactions.
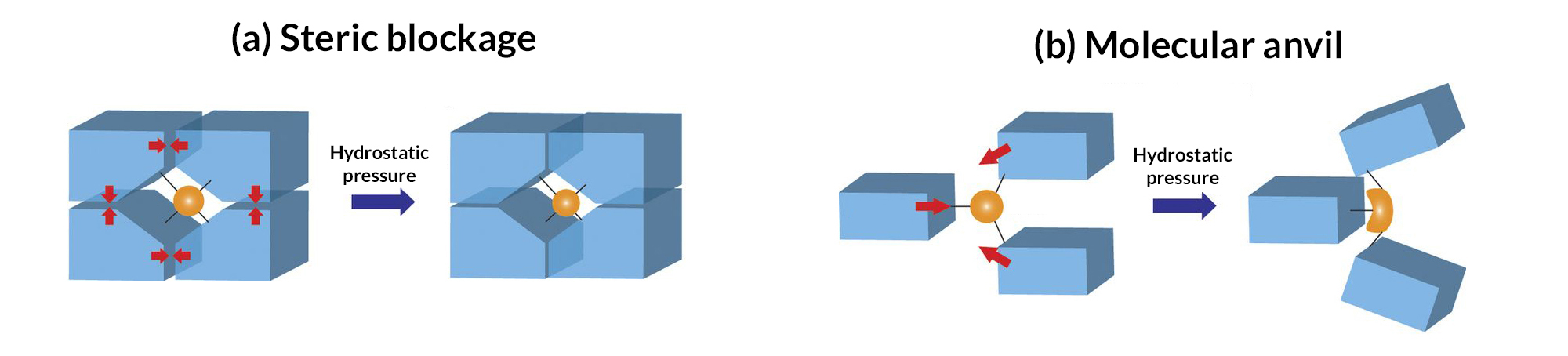
Characterization tool kit
To determine whether the complexes under study were in a hard-packed “steric blockage” configuration or a sparsely packed “molecular anvil” configuration, the researchers used small-molecule crystallography at ALS Beamline 11.3.1 (now moved to Beamline 12.2.1). They also performed in situ high-pressure powder diffraction measurements at Beamline 12.2.2 to monitor structural changes in the samples as the pressure increased from ambient up to 20 GPa.
In addition, the researchers performed x-ray absorption measurements as a function of pressure at the Advanced Photon Source (APS), to learn the valence state (electron distribution) of the metal centers. They also used computational tools to calculate how the structure should change under a given pressure.
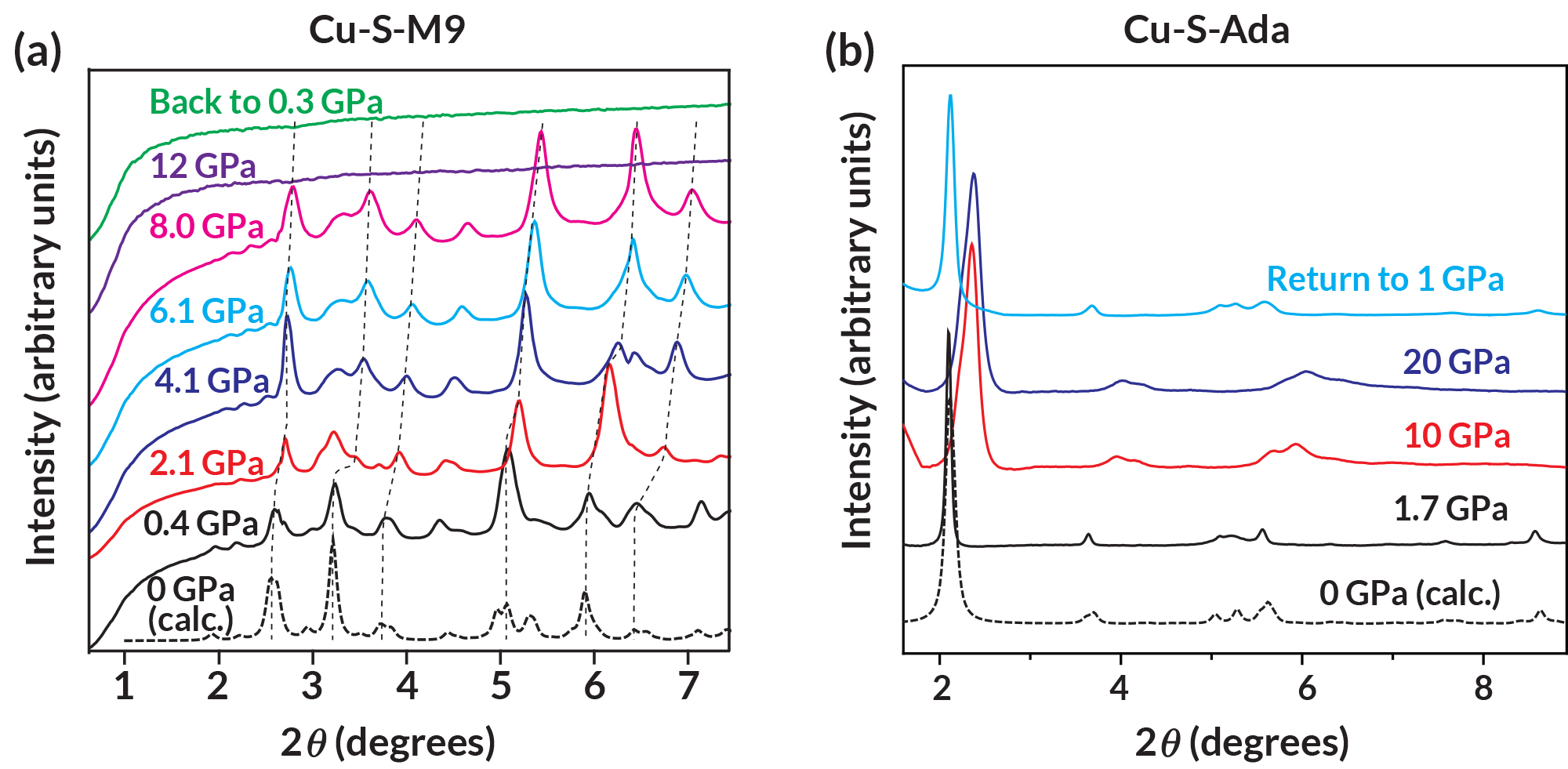
New mechanochemistry pathways
In general, the results showed that, at pressures around 8–12 GPa, the copper atoms in the sample were reduced—they underwent a chemical reaction in which they gained an electron from neighboring sulfur molecules. The copper atoms were then released from the complex, eventually agglomerating into nanoparticles. In contrast, a heat-driven reaction involving the same reactants would yield cuprous sulfide, not elemental copper.
The researchers are planning to explore the application of mechanochemistry to reactions that require extreme conditions (high heat or strong light) and/or do not yield the desired products—the reduction of nitrogen into ammonium, or the reduction of carbon dioxide into fuels, for example. Another beneficiary could be small-molecule drug discovery, where chiral selectivity is a big challenge. The work overall opens up a whole new landscape for synthesis that is both energy efficient and, because it doesn’t need heat or solvents, environmentally friendly as well.
Contact: Hao Yan
Researchers: H. Yan, F. Yang, F.H. Li, W.L. Mao, Z.-X. Shen, and N.A. Melosh (SLAC National Accelerator Laboratory and Stanford Univ.); D. Pan (Hong Kong Univ. of Science and Technology); Y. Lin, J.E.P. Dahl, and R.M.K. Carlson (SLAC); J.N. Hohman (Berkeley Lab); D. Solis-Ibarra (National Autonomous Univ. of Mexico); B.A. Tkachenko and P.R. Schreiner (Justus-Liebig Univ., Germany); A.A. Fokin (Justus Liebig Univ. and Igor Sikorsky Kiev Polytechnic Institute, Ukraine); and G. Galli (Univ. of Chicago).
Funding: Hong Kong Research Grants Council, National Natural Science Foundation (China), Alfred P. Sloan Foundation, Support Program for Research Projects and Technological Innovation (PAPIIT, Mexico), National Council for Science and Technology (CONACYT, Mexico), National Science Foundation, and the U.S. Department of Energy, Office of Science, Basic Energy Sciences Program (DOE BES). Operation of the ALS is supported by DOE BES.
Publications: H. Yan, F. Yang, D. Pan, Y. Lin, J.N. Hohman, D. Solis-Ibarra, F.H. Li, J.E.P. Dahl, R.M.K. Carlson, B.A. Tkachenko, A.A. Fokin, P.R. Schreiner, G. Galli, W.L. Mao, Z.-X. Shen, and N.A. Melosh, “Sterically controlled mechanochemistry under hydrostatic pressure,” Nature 554, 505 (2018), doi:10.1038/nature25765, and H. Yan, J.N. Hohman, F.H. Li, C. Jia, D. Solis-Ibarra, B. Wu, J.E.P. Dahl, R.M.K. Carlson, B.A. Tkachenko, A.A. Fokin, P.R. Schreiner, A. Vailionis, T.R. Kim, T.P. Devereaux, Z.-X. Shen, and N.A. Melosh, “Hybrid metal–organic chalcogenide nanowires with electrically conductive inorganic core through diamondoid-directed assembly,” Nat. Mater. 16, 349 (2017), doi:10.1038/nmat4823.
Adapted from the Stanford University press release, “In a First, Tiny Diamond Anvils Trigger Chemical Reactions by Squeezing.”
ALS SCIENCE HIGHLIGHT #377