SCIENTIFIC ACHIEVEMENT
X-ray analyses at the Advanced Light Source (ALS) provided key insights into the copper uptake mechanisms in a new organic-inorganic hybrid material that quickly and selectively removes copper ions from water.
SIGNIFICANCE AND IMPACT
The material provides an efficient tool for copper remediation as well as a blueprint for creating other hybrid materials for removing toxic metals from water.
Water remediation challenges
We rely on water to quench thirst and irrigate bountiful farmland. But what do you do when that once pristine water is polluted with wastewater from abandoned copper mines? A promising solution relies on materials that can adsorb heavy metals from wastewater. However, commercially available materials still lack the chemical specificity and load capacity to precisely separate copper ions from water.
Now, a team of scientists has designed a new crystalline material—called ZIOS (zinc imidazole salicylaldoxime)—that targets and traps copper ions from wastewater with unprecedented precision and speed. The scientists say that ZIOS offers the water industry and the research community the first blueprint for a water-remediation technology that scavenges specific heavy metal ions with a measure of control at the atomic level, which far surpasses the current state of the art.
ZIOS—inspired by nature
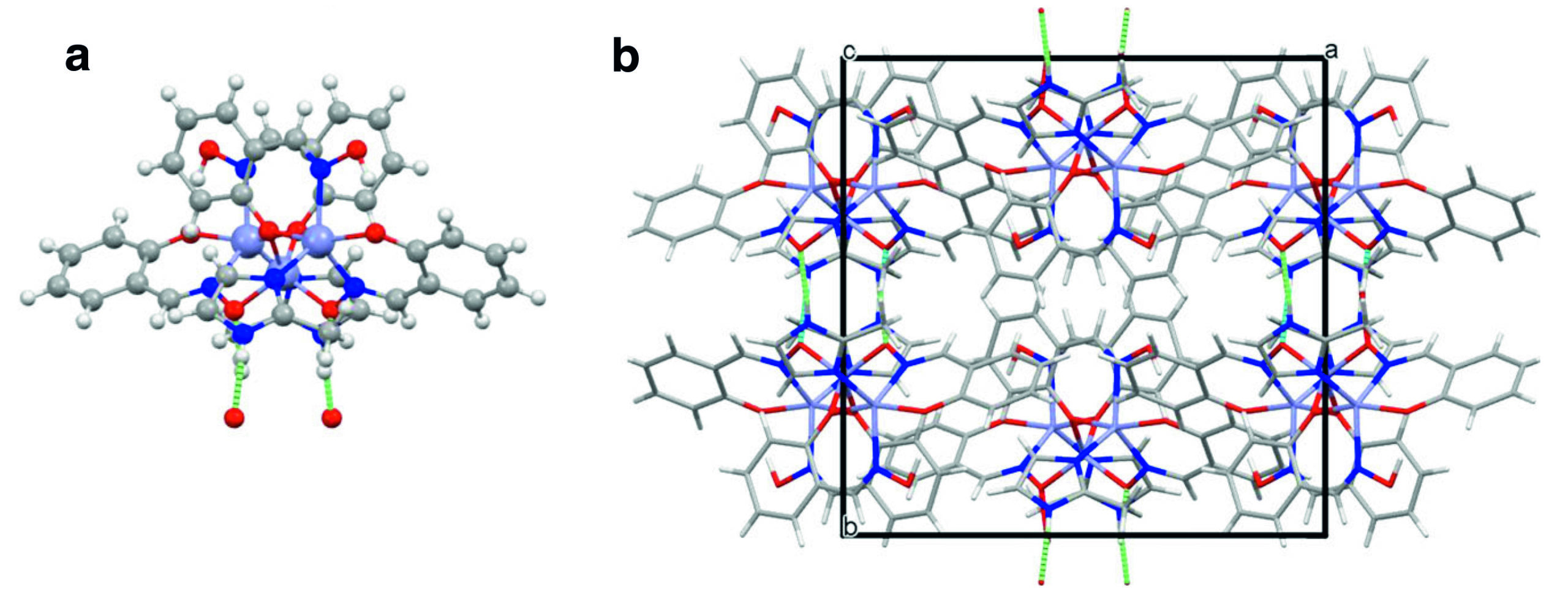
In designing ZIOS, researchers from Berkeley Lab’s Molecular Foundry were inspired by the way some copper-binding proteins use ring-shaped organic molecules (imidazoles) to capture copper ions in active sites. To mimic this, the researchers incorporated imidazole groups into supramolecular coordination complexes—large multimolecular complexes in which organic molecules are bound to a central metal atom (or in this case, three zinc atoms). At ALS Beamline 12.2.1, single-crystal x-ray diffraction confirmed that ZIOS is “trinuclear,” with channels about 2.7 Å wide, close to the typical length of a solid-state hydrogen bond (2–3 Å).
Synthesized through a simple and scalable process, ZIOS is highly stable in water and can remove copper ions under low-pH conditions (acidic mine drainage, for example), where other metal–organic frameworks (MOFs) are ineffective. Most intriguingly, ZIOS adsorbs copper ions 30 to 50 times faster than state-of-the-art copper adsorbents such as ZIF-8, a MOF that is much more porous.
Spectroscopic clues to the uptake mechanism
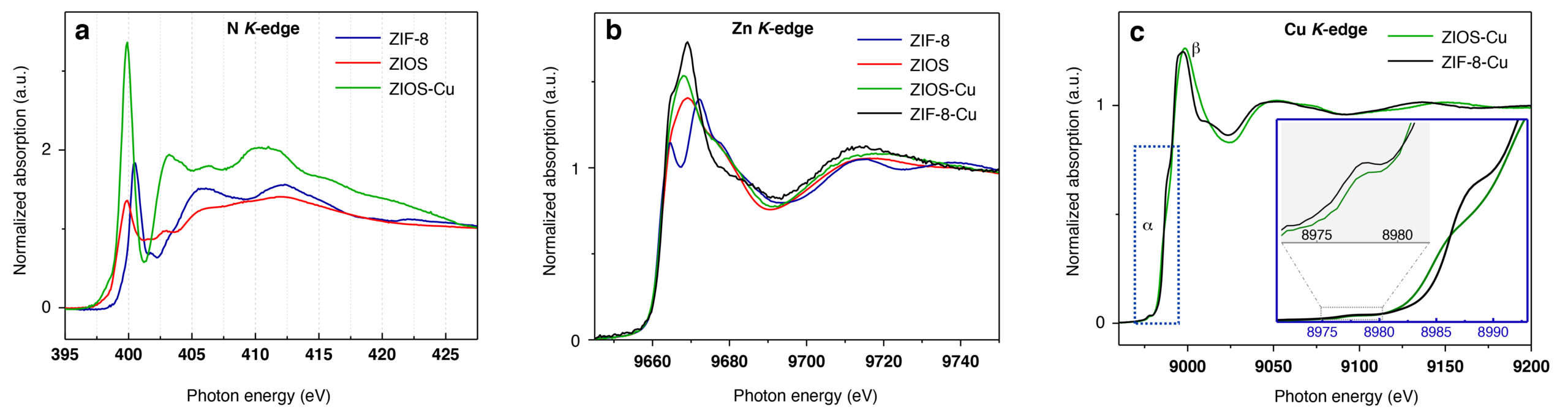
To better understand the mechanisms leading to copper-ion uptake in ZIOS and ZIF-8, the researchers utilized x-ray spectroscopy, including near-edge x-ray absorption fine-structure (NEXAFS) spectroscopy at ALS Beamline 6.3.2, to characterize the materials before and after adsorption of copper ions. In NEXAFS spectra, the energies and intensities of the peaks are typically sensitive to the local symmetry of the metal ions, the metal coordination number, and oxidation state changes. Analysis of the data led the researchers to hypothesize that ZIOS’s nanochannels expand when immersed in water, opening up pores primed to accept copper ions.
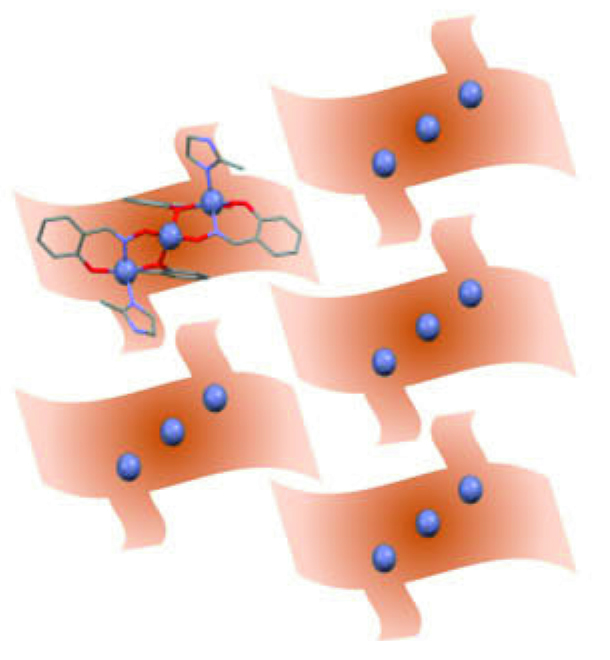
The breathing behavior of ZIOS
To support their hypothesis, the researchers performed molecular dynamics simulations, which confirmed that immersion of ZIOS unit cells in water indeed leads to expansion of the crystal lattice and the formation of nanochannels, through the propagation of a hydrogen-bonding network. Furthermore, when water is removed from the material, the crystal lattice structure contracts to its original size. Further experimental approaches will be necessary to observe this “breathing” behavior in operando.
Materials such as ZIOS will be critically important in lowering the cost and energy of water treatment, and studies such as this provide a blueprint for durable materials that can selectively remove of a variety of toxic metals from water.
Contact: Jeffrey Urban
Researchers: N.T. Bui, E.W. Zaia, C. Dun, T.M. Mattox, P. Fiske, R. Kostecki, and J.J. Urban (Berkeley Lab); H. Kang and J.R. Long (Berkeley Lab and UC Berkeley); S.J. Teat, G.M. Su, Y.S. Liu, and J. Guo (ALS); C.‑W. Pao and J.‑L. Chen (National Synchrotron Radiation Research Center, Taiwan); and K.R. Meihaus (UC Berkeley).
Funding: Berkeley Lab (Laboratory Directed Research and Development Program, Molecular Foundry) and the U.S. Department of Energy (DOE), Office of Energy Efficiency and Renewable Energy, Geothermal Technologies Office. Operation of the ALS is supported by the DOE Office of Science, Basic Energy Sciences program.
Publication: N.T. Bui, H. Kang, S.J. Teat, G.M. Su, C.‑W. Pao, Y.S. Liu, E.W. Zaia, J. Guo, J.‑L. Chen, K.R. Meihaus, C. Dun, T.M. Mattox, J.R. Long, P. Fiske, R. Kostecki, and J.J. Urban, “A nature-inspired hydrogen-bonded supramolecular complex for selective copper ion removal from water,” Nat. Commun. 11, 3947 (2020), doi:10.1038/s41467-020-17757-6.
Adapted from the Berkeley Lab news release, “Scientists Design New Framework for Clean Water.”
ALS SCIENCE HIGHLIGHT #434