SCIENTIFIC ACHIEVEMENT
A collaboration of university and industry researchers used x-ray crystallography at the Advanced Light Source (ALS) to investigate how the chikungunya virus, which can cause debilitating joint pain, engages a receptor protein found on the surfaces of joint cells.
SIGNIFICANCE AND IMPACT
The work provides a path forward in the fight against a family of viruses that can result in acute and chronic arthritis.

The chikungunya alphavirus
Chikungunya is a mosquito-borne disease that was first identified in 1952 in southern Tanzania. The disease, whose name means “to become contorted” in the region’s Makonde language, causes fever and acute joint pain that often resolves in about a week. However, up to half of chikungunya patients can develop chronic arthritis that persists for months or years. Chikungunya has now been identified in Asia, Africa, Europe, and the Americas, including the continental United States.
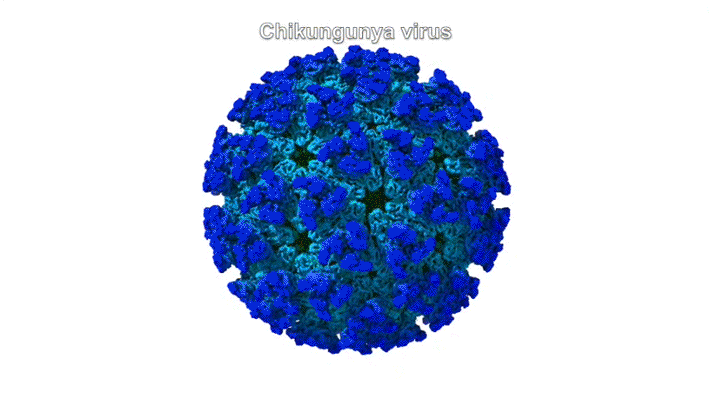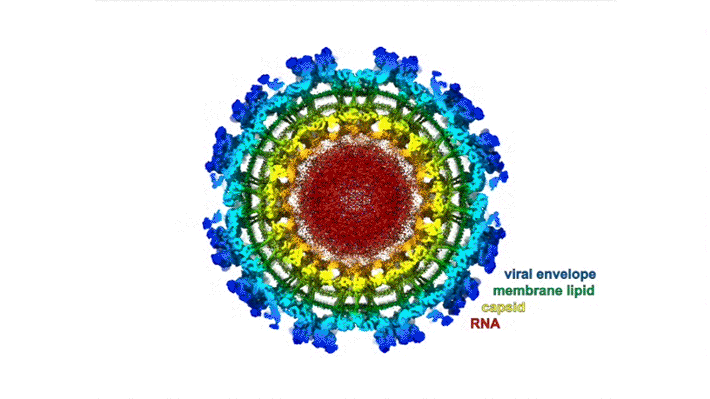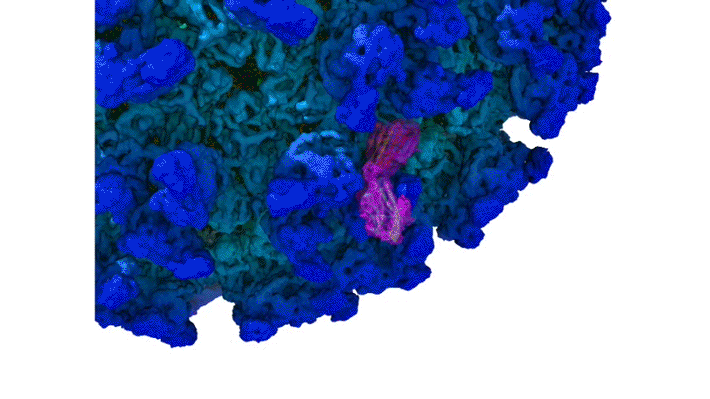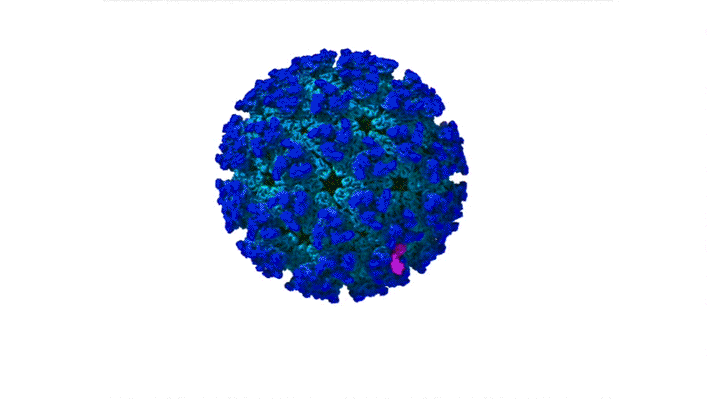
The chikungunya virus (CHIKV) belongs to a family of alphaviruses—enveloped viruses with a particular type of single-stranded RNA—that are spread by mosquitoes and cause debilitating musculoskeletal disease. Despite their epidemic potential, there are no approved vaccines or therapeutics for alphaviruses, and little is known about the molecular basis of alphavirus infection.
Identification of the receptor
In previous work, researchers identified a cell-adhesion protein, Mxra8, as the “molecular handle” that multiple alphaviruses latch onto in order to gain entry to skeletal muscle cells, fibroblasts (cells found in connective tissue), and chondrocytes (cells found in cartilage). Deletion of the Mxra8 gene in mice significantly reduced CHIKV infection and disease. Biochemical and biophysical experiments showed that Mxra8 directly binds to CHIKV and that this interaction can be prevented by using blocking antibodies or decoy receptors. However, to develop therapeutics against emerging pathogens, it is critical to understand how the pathogen interacts with the host and to know the exact footprint of this interaction.
Structure of CHIKV–Mxra8 Complex
In this work, the researchers performed x-ray crystallography and cryogenic electron microscopy (cryo-EM) studies of Mxra8 bound to CHIKV. The crystallography experiments (using non-infectious CHIKV particles) were done remotely, using ALS Beamline 4.2.2, part of the Molecular Biology Consortium (MBC). Cryo-EM was performed at the Washington University Center for Cellular Imaging (WUCCI). The combination of a high-resolution (2.2 Å) Mxra8 crystal structure and the cryo-EM CHIKV–Mxra8 reconstruction (4 to 5 Å) allowed the researchers to build a detailed atomic model of Mxra8 bound to CHIKV. The ability to remotely collect diffraction data and the excellent support from on-site beamline staff were critical for this study.
An unusual binding architecture
Mature alphaviruses contain a lipid bilayer with 240 embedded heterodimers assembled into 80 trimeric spikes. The researchers’ analysis showed that the Mxra8 receptor binds to CHIKV by wedging into a cleft formed in one spike while also engaging a neighboring spike. Also, the researchers had predicted that the Mxra8 receptor would contain two standard antibody-like domains. However, the high-resolution crystal structure surprisingly revealed a novel arrangement in which the antibody-like domains are oriented head-to-head, with one domain inserted into a loop of the second domain. To the researchers’ knowledge, the only protein with a similar architecture is an artificially engineered antibody-like monomer.
As alphaviruses infect a wide range of hosts, the researchers are continuing to explore the structural basis of alphavirus engagement with Mxra8 equivalents in cattle and other mammals. The insights provided by such studies could form the basis for therapies and improved vaccine designs that could mitigate the disease-causing effects of multiple emerging alphaviruses.
Contact: Katherine Basore
Researchers: K. Basore, A.S. Kim, C.A. Nelson, R. Zhang, B.K. Smith, M. Cheng, M.L. Gross, M.S. Diamond, and D.H. Fremont (Washington University in St. Louis) and C. Uranga, L. Vang, and J. Smith (PaxVax Corporation).
Funding: National Institutes of Health. Operation of the ALS is supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences Program.
Publication: K. Basore, A.S. Kim, C.A. Nelson, R. Zhang, B.K. Smith, C. Uranga, L. Vang, M. Cheng, M.L. Gross, J. Smith, M.S. Diamond, and D.H. Fremont, “Cryo-EM Structure of Chikungunya Virus in Complex with the Mxra8 Receptor,” Cell 177, 1725 (2019), doi:10.1016/j.cell.2019.04.006.
ALS SCIENCE HIGHLIGHT #411