Rechargeable lithium-ion batteries have become the dominant power source for portable electronics and are now on the verge of enabling the widespread adoption of electric vehicles. Right now, tremendous research efforts are being devoted to increasing the capacity of layered transition-metal (TM) oxides—battery electrode materials of the form LiTMO2. This family of lithium-rich oxides demonstrates capacities far beyond what’s explained solely by TM redox (chemical reactions involving electron transfer between elements). Oxygen integrated into the material’s lattice is typically believed to play a role as well, but the precise nature of this participation is still not understood.
To probe the effect of the TM on oxygen redox activity, researchers synthesized two lithium-rich layered oxides, one with manganese (Mn) as the TM and the other with ruthenium (Ru). Comprehensive studies were performed to capture the lattice oxygen activity, combining a suite of soft x-ray spectroscopy techniques with in situ differential electrochemical mass spectrometry (DEMS).
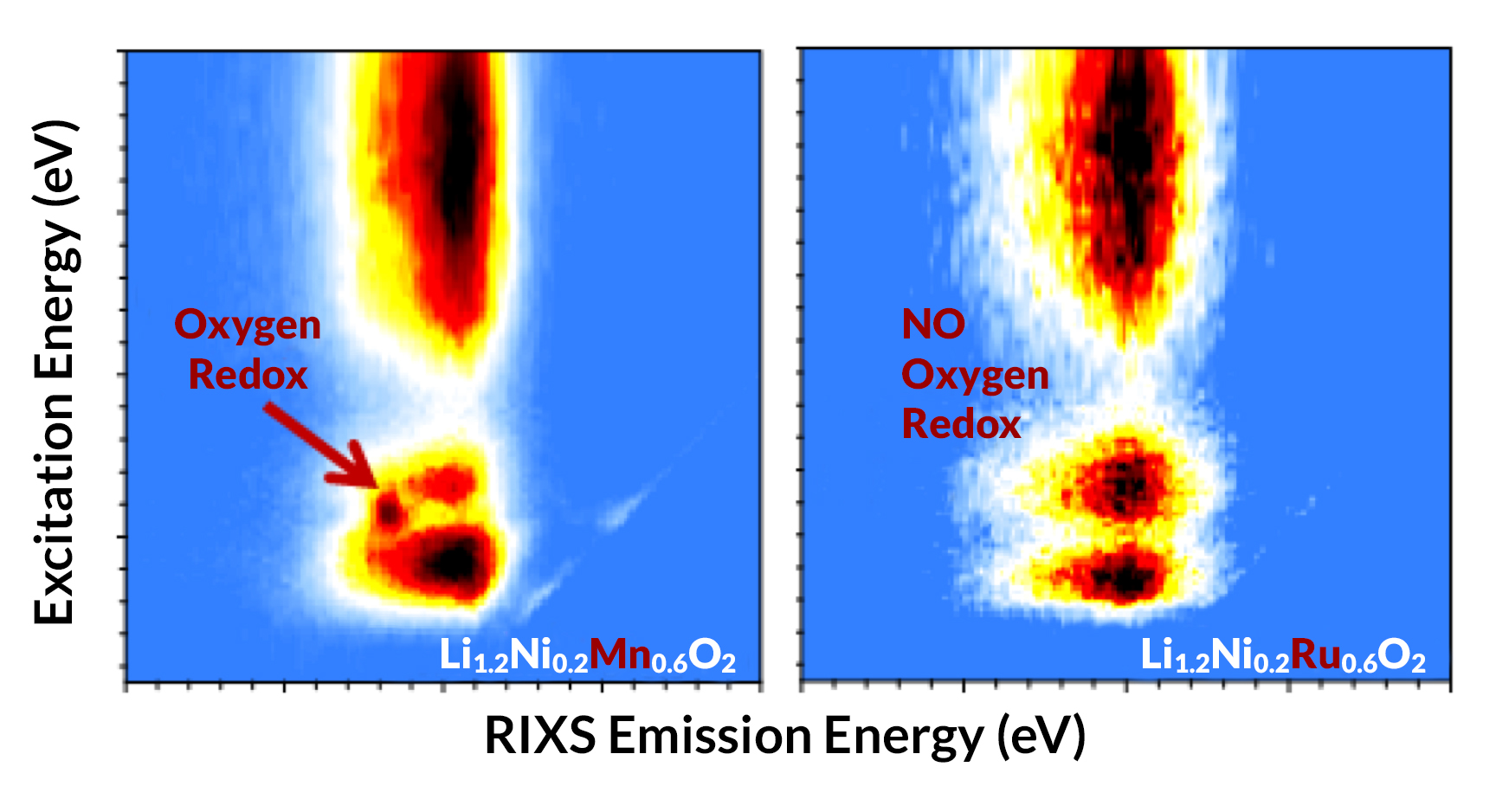
At the ALS, the excitation of lattice oxygen at various states of charge during battery operation was probed using high-efficiency, full-range mapping of resonant inelastic x-ray scattering (mRIXS) at Beamline 8.0.1. The results showed distinct oxygen behavior in the two materials—direct experimental evidence of strong oxygen redox in the Mn-based material, but not in the Ru-based one. This finding provides new insight into the complex oxygen redox mechanism and its effect on the development of advanced high-capacity lithium-ion cathodes. Moreover, the work is an explicit example of the employment of mRIXS in understanding lattice oxygen redox processes for energy storage and conversion applications.
J. Xu, M. Sun, R. Qiao, S.E. Renfrew, L. Ma, T. Wu, S. Hwang, D. Nordlund, D. Su, K. Amine, J. Lu, B.D. McCloskey, W. Yang, and Wei Tong, “Elucidating anionic oxygen activity in lithium-rich layered oxides,” Nat. Commun. 9, 947 (2018), doi:10.1038/s41467-018-03403-9.