Interest in hydrogen fuel for automotive applications has been growing steadily in the scientific and automotive community over the last decade. Recently, researchers working at the ALS and the Molecular Foundry developed a promising new materials recipe based on magnesium nanocrystals and graphene for a battery-like hydrogen fuel cell with improved performance in key areas.
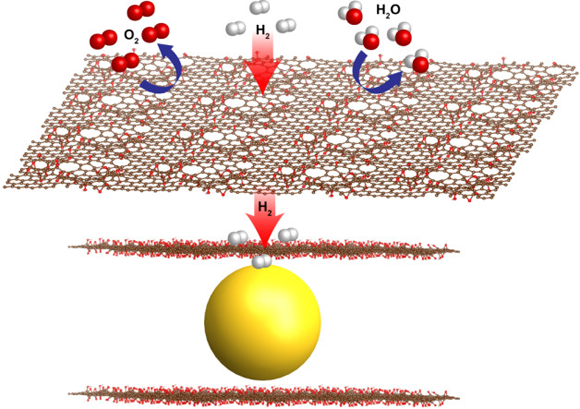
Hydrogen is the lightest and most plentiful element on Earth and could serve as a clean, carbon-free, virtually limitless energy source. However, safe hydrogen storage remains a formidable scientific challenge. Metal hydrides—chemical compounds containing a metal bonded to hydrogen—used to be the most popular solution, as they offer ample storage capacity and do not require cryogens or exceedingly high pressures for operation. However, hydrides have now largely been abandoned because their structures tend to break down in the presence of oxygen and they absorb and release hydrogen slowly.
The solution developed by ALS and Molecular Foundry scientists enables stable storage of hydrogen by wrapping hydrogen-absorbing magnesium nanocrystals with atomically thin graphene oxide sheets. The graphene shields the nanocrystals from oxygen, moisture, and contaminants, while tiny, natural holes in the graphene’s structure allow the smaller hydrogen molecules to pass through. This filtering process overcomes common problems degrading the performance of metal hydrides for hydrogen storage. These graphene-encapsulated magnesium crystals act as “sponges” for hydrogen, offering a very compact and safe way to take in and store hydrogen. The nanocrystals also permit faster fueling and reduce the overall “tank” size.
The tiny size of the graphene-encapsulated nanocrystals, which measure only about 3–4 nanometers, or billionths of a meter, across, is critical to the new fuel cell materials’ fast capture and release of hydrogen, as the nanocrystals have more surface area available for reactions than the same material would at larger sizes. The graphene also protects the magnesium from exposure to air, which would render it unusable for the fuel cell.
Working at the Molecular Foundry, researchers found a simple, scalable, and cost-effective “one-pan” technique to mix up the graphene sheets and magnesium oxide nanocrystals in the same batch. They then studied the coated nanocrystals’ structure using the x-ray absorption near-edge structure (XANES) technique at ALS Beamlines 8.0.1 and 4.0.3. XANES allowed the researchers to investigate different oxidation states of their samples and to distinguish the oxidation state near the surface and in the bulk. The oxidation state indicates whether the magnesium has fully absorbed hydrogen or not. ALS data showed that hydrogen gas pumped into the fuel cell mixture reacted with the magnesium nanocrystals to form a more stable molecule called magnesium hydride, while locking out oxygen from reaching the magnesium.
The results suggest the possibility of practical hydrogen storage and use in the future and represent a generally applicable approach to stabilizing reactive materials while still harnessing their unique activity—concepts that could have wide-ranging applications for batteries, catalysis, and energetic materials.
Next steps in the research will focus on using different types of catalysts—which can improve the speed and efficiency of chemical reactions—to further improve the fuel cell’s conversion of electrical current, and studying whether different types of material can also improve the fuel cell’s overall capacity.
Contact: Jeffrey Urban
Research conducted by: E.S. Cho, A.M. Ruminski, S. Aloni, and J.J. Urban (Molecular Foundry, Materials Sciences Division, Berkeley Lab); Y. Liu and J. Guo (ALS, Berkeley Lab).
Research funding: This research is part of a National Lab Consortium, dubbed HyMARC (Hydrogen Materials—Advanced Research Consortium) that seeks safer and more cost-effective hydrogen storage effort. Operation of the ALS and the Molecular Foundry are supported by the U.S. Department of Energy, Office of Basic Energy Sciences.
Publication about this research: E.S. Cho, A.M. Ruminski, S. Aloni, Y. Liu, J. Guo, J.J. Urban, “Graphene oxide/metal nanocrystal multilaminates as the atomic limit for safe and selective hydrogen storage,” Nature Communications 7, 10804 (2016).
ALS SCIENCE HIGHLIGHT # 332