SCIENTIFIC ACHIEVEMENT
The mechanisms that affect the regulation of cell growth in certain tumor cells were revealed by a Genentech study of enzyme structures, conducted in part at the Advanced Light Source (ALS).
SIGNIFICANCE AND IMPACT
The work establishes a framework for the rational discovery of new therapeutics to improve upon currently existing treatments for certain cancers.
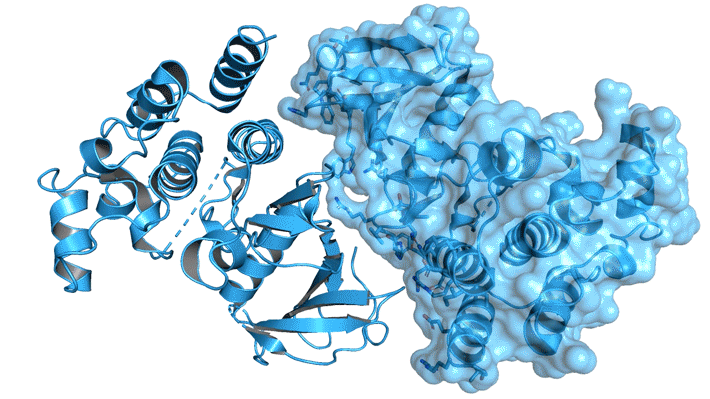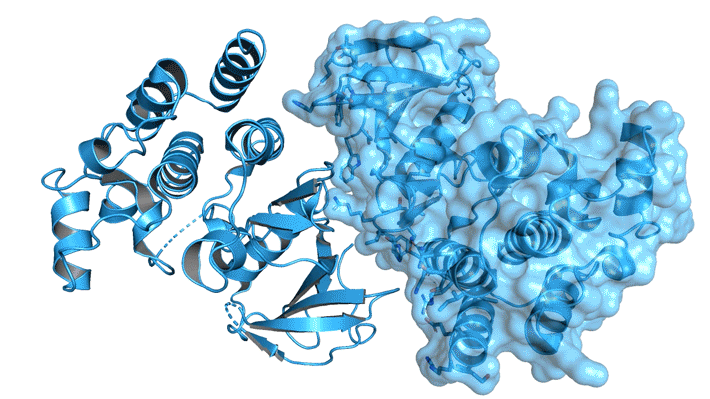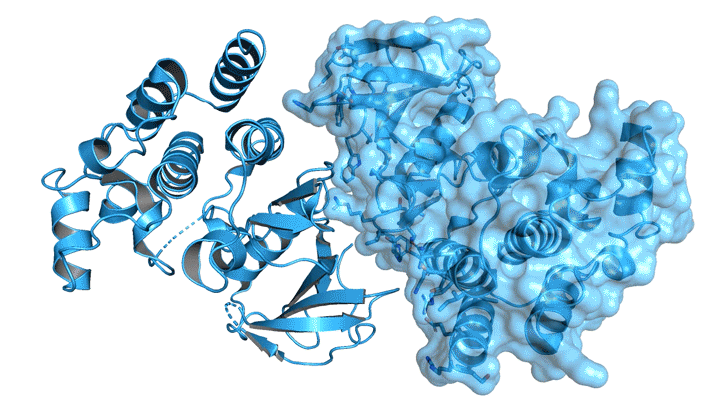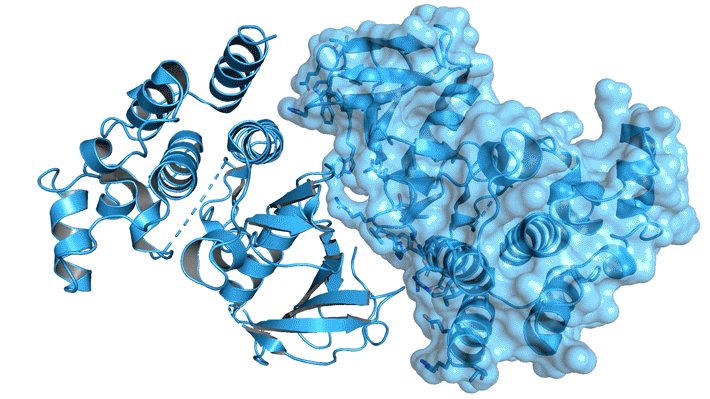
A cell-signaling cascade
One way cells regulate growth is through growth factors—molecules that bind on the outer surface of a cell and turn on a cascade of signals inside the cell. The signals are transmitted through a series of enzymes by phosphorylation (the addition of phosphoryl groups) inside the cell, ultimately leading to cell division and growth. While it’s advantageous for cells to respond to such signals when an organism is developing or healing, mutations in the pathway can cause the signal to get stuck in the “on” state, leading to out-of-control tumor growth. At Genentech, researchers have been studying this pathway to understand how oncogenic mutations shift the growth signal to the “on” state and how to dial it back, as a therapeutic intervention. Here, they focused on the BRAF enzyme in the cascade, which has been found to be mutated in many cancers, especially certain skin cancers.
Contradictory signals

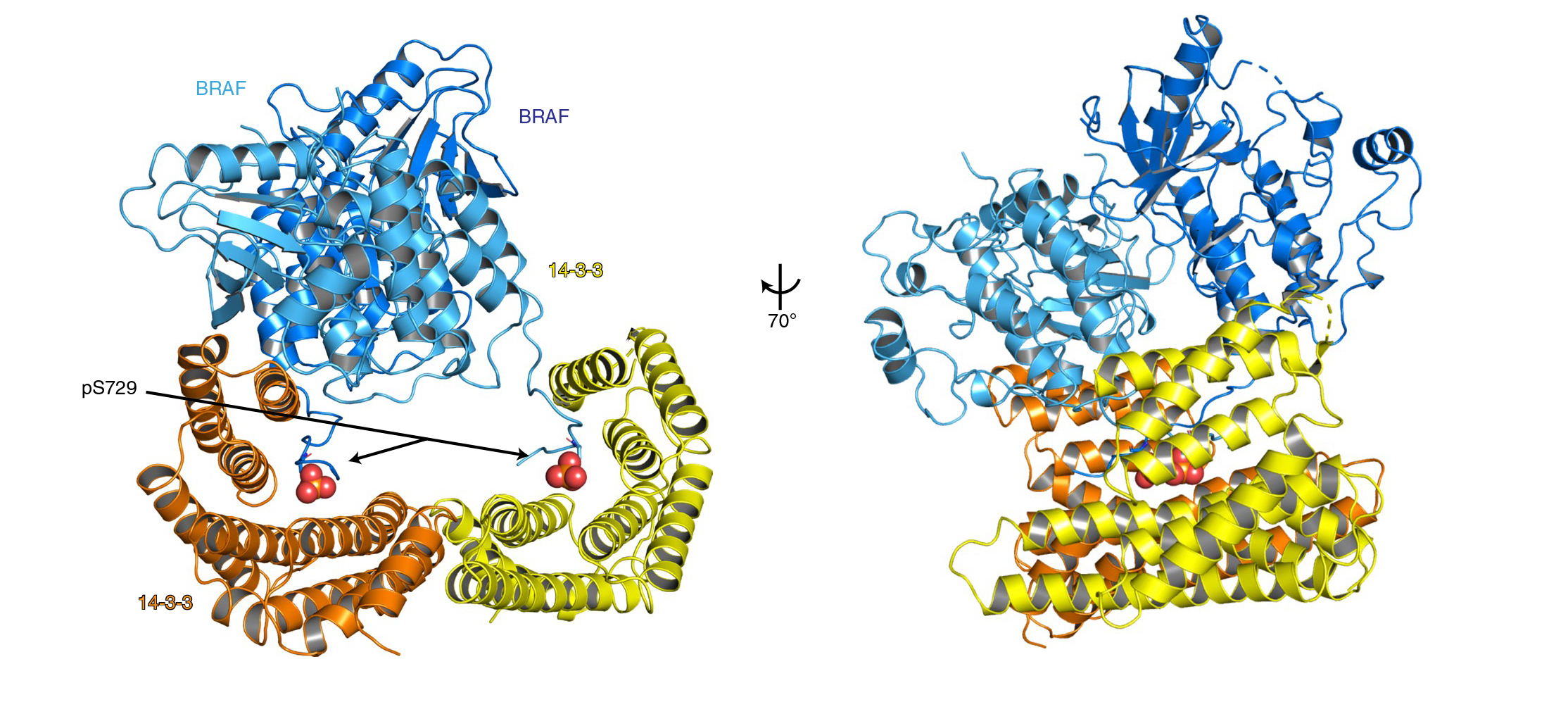
Transmission of the cell-growth signal requires the linking of two RAF molecules into a dimer. Therefore, anything that destabilizes RAF dimers could serve as an “off” signal. For example, the presence of adenosine triphosphate (ATP), a small molecule that drives many biological processes, disfavors the formation of RAF dimers. At the same time, however, ATP also supplies the phosphoryl groups that drive the phosphorylation cascade and is therefore necessary for switching the signal “on.” Since both RAF dimerization and ATP binding are necessary to activate the cascade, the two events appear to be working against each other. Genentech researchers hypothesized that certain “adaptor” molecules, known as 14-3-3 proteins, might counteract RAF destabilization by ATP.
Synchrotron-based crystallography
To test their hypothesis, the researchers performed protein crystallography at ALS Beamline 5.0.2 and Stanford Synchrotron Radiation Laboratory. They analyzed the structure of BRAF (a frequently mutated form of RAF) bound to 14-3-3. They also studied BRAF with and without the presence of ACP (a nonreactive surrogate for ATP).
ALS Beamline 5.0.2, part of the Berkeley Center for Structural Biology, is a cutting-edge, high-quality, well-staffed beamline that Genentech has relied on to collect hundreds, if not thousands, of data sets over many years. This long-term partnership enables Genentech scientists to routinely send their one-of-a-kind protein crystals for analysis, confident that their chances of acquiring good data will be maximized.
A stabilizing scaffold
The results revealed how interactions between BRAF and ATP induce an inactive (monomeric) form of BRAF by shifting portions of the dimer interfaces away from each other when there is no active signal to turn on the cascade. In addition, the BRAF–14-3-3 structures showed that 14-3-3 forms a dimer that acts as a scaffold, stabilizing BRAF so that it too can dimerize, even in the presence of ATP, once the pathway is activated. Analysis of the structures also suggests that most oncogenic BRAF mutations counteract the growth-inhibiting effects of ATP by lowering the threshold for BRAF dimerization and shifting the equilibrium toward the BRAF dimer.
Such insights will help researchers perform rational drug discovery and, eventually, to match treatments to patients with tumors caused by specific mutations (i.e., personalized medicine). More generally, by better understanding the cellular context of these important signaling pathways, researchers will be better prepared to identify and develop the next generation of therapeutics that will be needed against constantly evolving cancer targets.
Contacts: Sarah Hymowitz and Jawahar Sudhamsu
Researchers: N.P.D. Liau, T.J. Wendorff, J.G. Quinn, M. Steffek, W. Phung, P. Liu, J. Tang, F.J. Irudayanathan, S. Izadi, A.S. Shaw, S. Malek, S.G. Hymowitz, and J. Sudhamsu (Genentech Inc.).
Funding: Genentech Inc. and National Institutes of Health. Operation of the ALS is supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences program (DOE BES).
Publication: N.P.D. Liau, T.J. Wendorff, J.G. Quinn, M. Steffek, W. Phung, P. Liu, J. Tang, F.J. Irudayanathan, S. Izadi, A.S. Shaw, S. Malek, S.G. Hymowitz, and J. Sudhamsu, “Negative regulation of RAF kinase activity by ATP is overcome by 14-3-3-induced dimerization,” Nat. Struct. Mol. Biol. 27, 134 (2020), doi:10.1038/s41594-019-0365-0.
ALS SCIENCE HIGHLIGHT #420