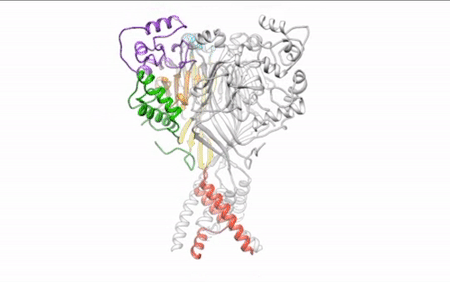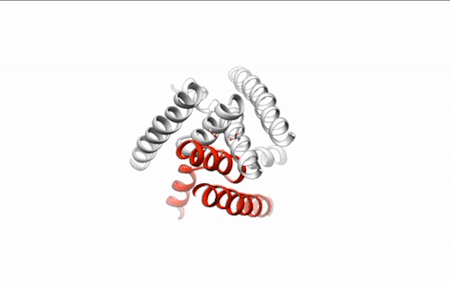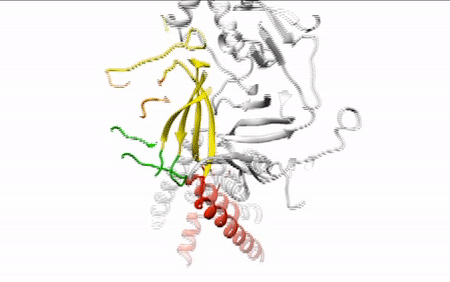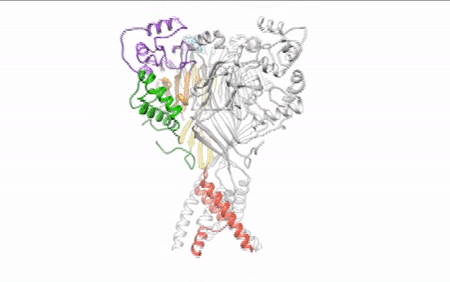
Our daily function depends on signals traveling between nerve cells (neurons) along fine-tuned pathways. Central nervous system neurons contain acid-sensing ion channel 1a (ASIC1a), a protein important in sensing pain and forming memories of fear. An ion channel lodged in the cell membrane that provides a pathway for sodium ions to enter the cell, ASIC1a opens and closes in response to changes in extracellular proton concentrations. When protons accumulate outside the neuron, the channel opens, allowing sodium ions to flow into the cell, depolarizing the cell membrane and generating an electrical signal. The channel eventually becomes desensitized to protons and the gate closes. Scientists have visualized both the open and desensitized channel structures, but the third structure, which forms when the protons dissipate and the channel closes, remained elusive. Using protein crystallography at the ALS, researchers finally visualized the closed channel.
Researchers used protein crystals of ASIC1a channels purified in the closed state. Compiling x-ray data from ALS Beamline 5.0.2 and the Advanced Photon Source, they built a model of the closed channel structure. New model in hand, they compared structures of open and closed channels and found areas of ASIC1a that shift to allow signals through the cell membrane at the right time.
Defining the closed structure allowed researchers to generate a comprehensive molecular model of how ASIC1a toggles between the closed, open, and desensitized states. This insight into proton-dependent signaling in the central nervous system gives scientists a blueprint to develop therapeutics to modulate ASIC1a channels and pain response in neurons.
N. Yoder, C. Yoshioka, and E. Gouaux, “Gating mechanisms of acid-sensing ion channels,”Nature 555, 397–401 (2018), doi:10.1038/nature25782.