SCIENTIFIC ACHIEVEMENT
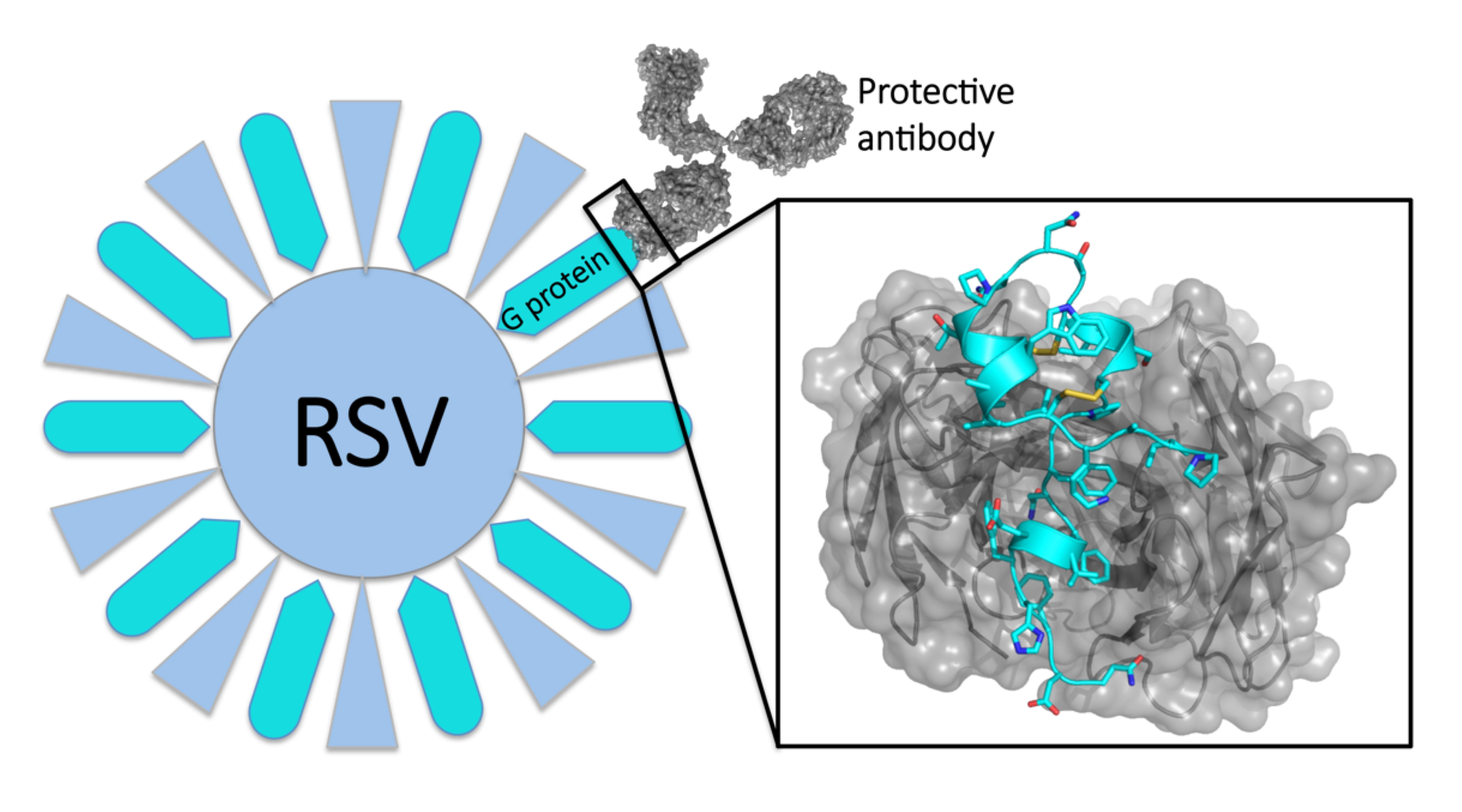
The molecular structures of human antibodies bound to a respiratory syncytial virus (RSV) surface protein reveal potential targets for antiviral therapies.
SIGNIFICANCE AND IMPACT
The work provides a promising route for designing a vaccine effective against a broad range of RSV strains, which cause serious respiratory disease in infants and older adults.
Principal investigator Rebecca DuBois (University of California, Santa Cruz) describes her work on a vaccine for respiratory syncytial virus (RSV). (Credit: A. Jones/UC Santa Cruz)
Vaccine failures prompt new approach
Respiratory syncytial virus (RSV) causes 118,000 deaths in young children each year worldwide and 57,000 hospitalizations of children each year in the United States alone. Attempts to develop a vaccine—at first based on inoculations with inactivated virus, and then targeting a key viral surface protein (RSV F)—have failed. Recent studies, however, suggest that a second viral surface protein (RSV G) is a promising target.
In one earlier study, elevated concentrations of antibodies targeting both RSV F and RSV G were associated with lower clinical disease severity scores, despite a substantially lower absolute abundance of anti-G antibodies. Another study found that higher levels of maternal anti-G antibodies correlated with less severe disease in infants (up to 4–6 months of age), even though anti-G antibodies were again found in lower concentrations.
While the RSV F surface protein allows the virus to enter a host cell, RSV G is what allows the virus to stick to lung cells and distort immune responses. This disruption of the immune response by RSV G might explain why vaccines based on RSV F have failed. Thus, a protective antibody response that blocks RSV G activity could be more effective, but scientists didn’t know how antibodies target RSV G at the molecular level.
Reverse vaccinology
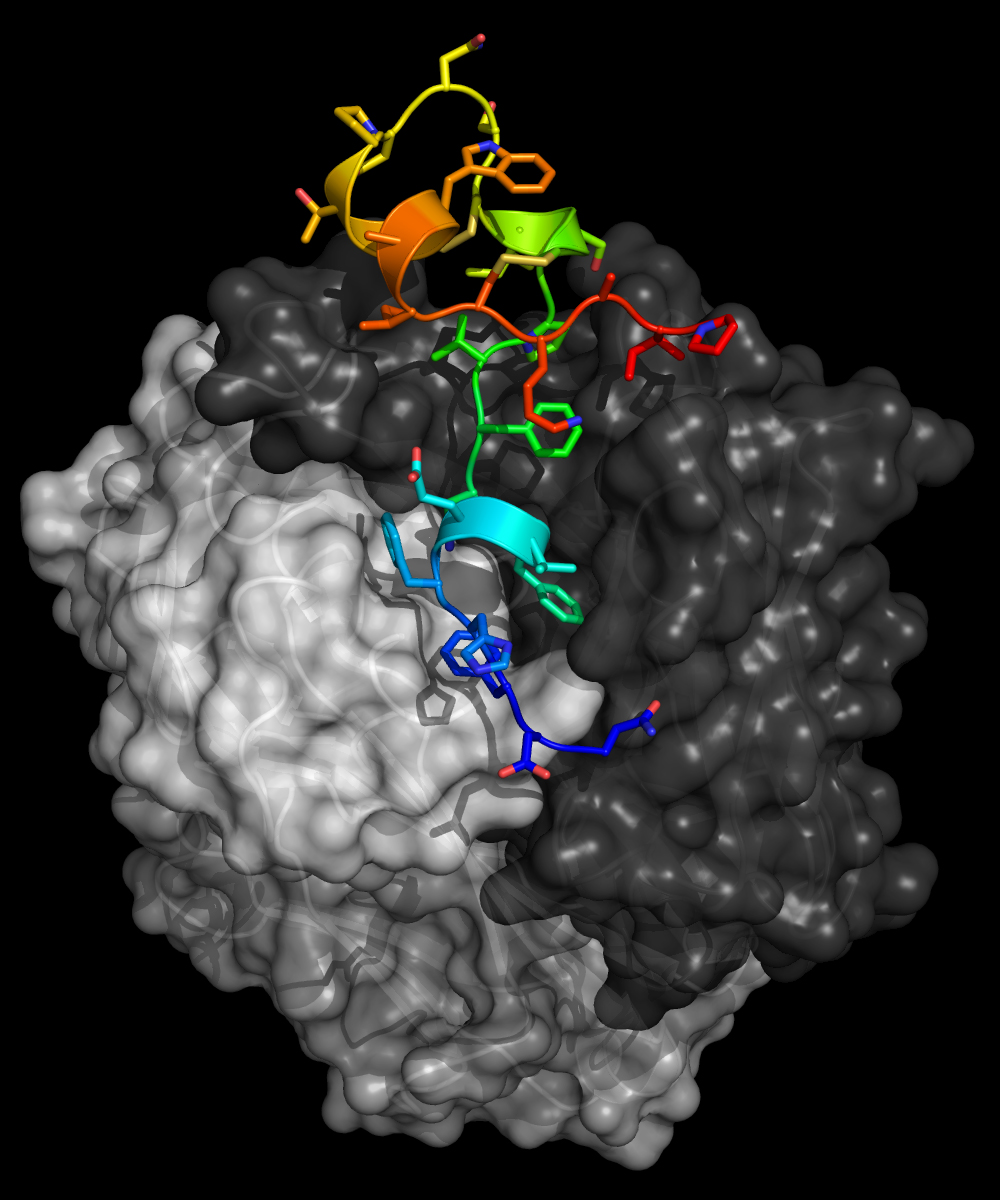
In this work, the researchers took a “reverse vaccinology” approach: they are first studying where and how the antibodies bind to the virus, so that they can reverse engineer a vaccine antigen (a substance that induces an immune response upon vaccination).
Collaborators at Trellis Bioscience, a biotechnology company in Menlo Park, California, successfully isolated anti-G antibodies from patients recently infected with RSV. The research team then co-crystallized the protective antibodies with RSV G protein, producing submillimeter-sized crystals.
X-ray protein crystallography of the samples at ALS Beamline 8.3.1 was used to determine the high-resolution molecular structures, allowing the researchers to pinpoint the exact sites on the virus targeted by the anti-G antibodies.
Region of vulnerability
The structures revealed that the anti-G antibodies bind with high affinity to a three-dimensional, twisted sequence of amino acids called the central conserved domain (CCD)—a region of vulnerability on the RSV G protein. Because this domain is the same in all strains of the virus, the protective antibodies are broadly neutralizing: exactly the type of antibodies that are the desirable products of successful vaccination.
The study also revealed that the protective antibodies block the ability of RSV G to bind and induce signaling in a receptor (CX3CR1) that’s involved in attaching the virus to lung cells and distorting the immune response. Thus, the antibodies have the dual protective functions of blocking virus infection as well as blocking virus manipulation of human immune responses.
Based on these results, Trellis Bioscience is currently developing an antiviral antibody therapy heading toward clinical trials. Going forward, the researchers will be studying how other protective human antibodies bind to RSV G in order to design novel vaccine antigens, with the long-term goal of creating the first effective RSV vaccine.

Contact: Rebecca DuBois
Researchers: S.O. Fedechkin, N.L. George, J.T. Wolff, and R.M. DuBois (UC Santa Cruz) and L.M. Kauvar (Trellis Bioscience LLC).
Funding: National Institutes of Health; University of California Office of the President, Multicampus Research Programs and Initiatives; and Program for Breakthrough Biomedical Research. Operation of the ALS is supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences Program (DOE BES).
Publication: S.O. Fedechkin, N.L. George, J.T. Wolff, L.M. Kauvar, and R.M. DuBois, “Structures of respiratory syncytial virus G antigen bound to broadly neutralizing antibodies,” Sci. Immunol. 3, eaar3534 (2018), doi:10.1126/sciimmunol.aar3534.
Adapted from the UC Santa Cruz press release, “Antigen study supports new approach to vaccine for respiratory syncytial virus.”
ALS SCIENCE HIGHLIGHT #374