SCIENTIFIC ACHIEVEMENT
Researchers genetically engineered mammalian cells to produce their own magnetic “handles” and revealed their magnetic, physical, and chemical properties, measured in part at the Advanced Light Source (ALS).
SIGNIFICANCE AND IMPACT
The work provides a foundation for future bioengineering efforts aimed at enabling genetically controlled magnetic manipulation of molecular processes in living mammalian cells.
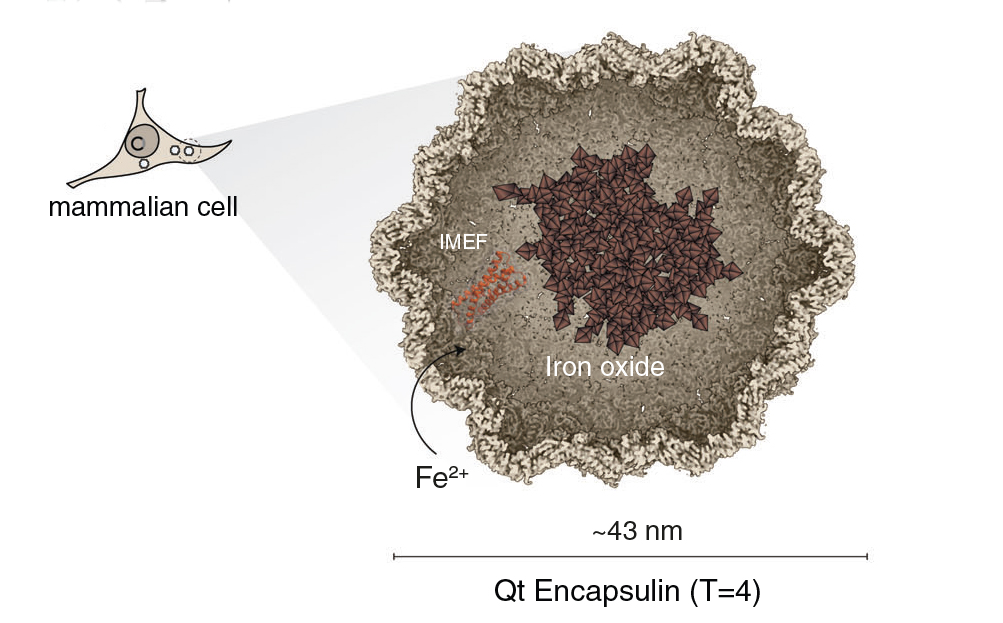
An internal compass for cells
Some bacteria have evolved the remarkable ability to align themselves with Earth’s magnetic field, owing to self-synthesized chains of magnetic nanocrystals that provide them with an internal compass needle. It’s thought that following magnetic field lines helps these single-celled organisms propel themselves toward optimal (for them) environments.
Multicellular organisms—including humans—can also benefit from cellular compasses, or magnetic “handles,” to maneuver cells as needed. Possible future applications include cell sorting, cell tracking (and imaging), and targeted drug delivery. However, the genetic programming that allows bacteria to natively produce magnetic organelles is lacking in mammals, and attempts to introduce magnetic agents into mammalian cells are stymied by cellular defense mechanisms.
To get around this, a large multinational research collaboration based in Germany genetically modified mammalian cells to self-produce protein nanocompartments in which iron oxides can be created and stored. The group then characterized the magnetic, physical, and chemical properties of the nanocompartment cargo and demonstrated the ability to manipulate the resulting live engineered cells using magnetic fields.
Sample synthesis and analysis
The researchers introduced into mammalian cells a set of genetic constructs (engineered DNA) for the overexpression of encapsulin, the protein building block of nanocompartment shells produced by Quasibacillus thermotolerans bacteria. Also included were constructs for a red fluorescent protein for detection purposes and a ferroxidase, an enzyme that promotes the oxidation of reactive Fe2+ to more stable Fe3+ (to facilitate iron oxide accumulation). Cellular uptake of Fe2+ was enhanced by co-expression of a protein that transports iron into cells. Finally, to provide a source of iron, the cell medium was supplemented with ferrous ammonium sulfate.
After 72 hours, the modified cells were sorted using magnetic-activated cell sorting (MACS) columns. Within the cell fraction retained in the MACS columns, the researchers discovered encapsulin shells that contained ultrafine (1–3 nm) quasicrystalline ferric oxide/hydroxide cores that exhibited ferrimagnetism and paramagnetism. However, determining the precise identity of the magnetic particles required the ability to distinguish between different species of iron oxide at the scale of individual particles.
Ironing out the iron species
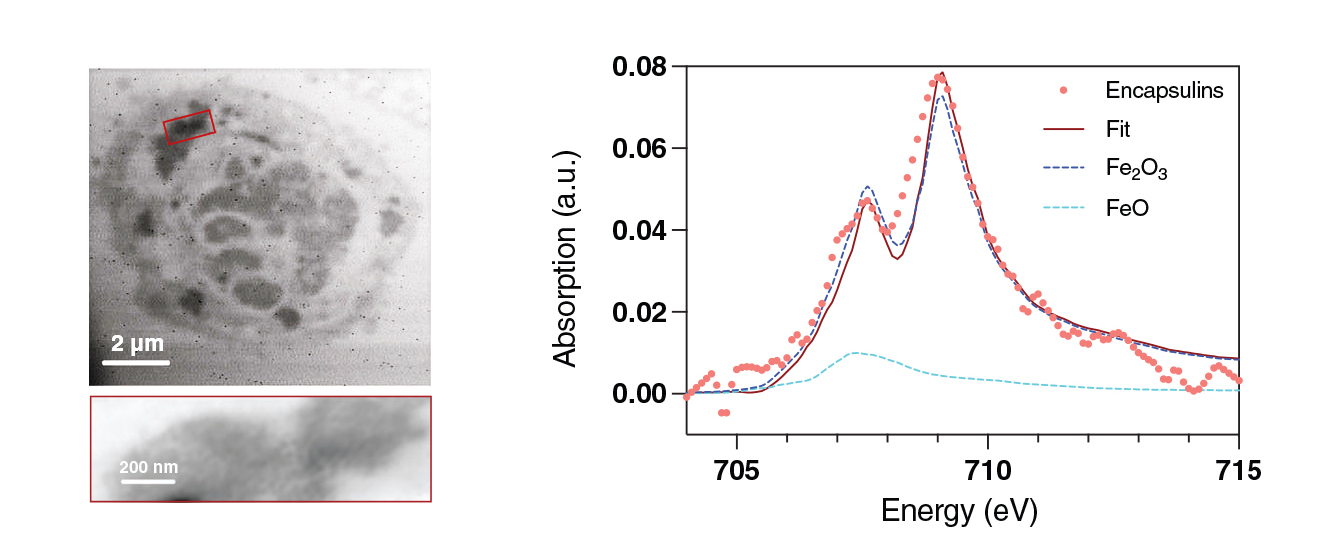
To gain insight into the local coordination and chemical state of the iron in the encapsulin cores, the researchers analyzed ultrathin sections of MACS-retained cells using high-resolution scanning transmission x-ray microscopy (STXM) and x-ray absorption spectroscopy (XAS) at ALS Beamline 11.0.2.
These methods, in combination with supporting Raman spectroscopy, were instrumental in determining that the most parsimonious explanation for the ferri- and paramagnetism measured in the MACS-retained cells is that the encapsulin cores are composed of a quasicrystalline maghemite phase (γ-Fe2O3) embedded within an amorphous iron-oxide-phosphorous matrix. Of the two main forms of ferric oxide (Fe2O3)—hematite (α-Fe2O3) and maghemite (γ-Fe2O3)—the latter has much stronger magnetic properties and is more compatible with the overall observations.
Finally, the researchers established that the MACS-retained cells can be manipulated to follow magnetic field lines around a magnetized needle and can also be regrown in patterns determined by magnetic gradients around bulk permanent magnets. Thus, this discovery provides a pathway by which cells can be engineered for control by external magnetic fields, opening up new possibilities for cell engineering and biomedical applications.
Contacts: Maria V. Efremova and Gil G. Westmeyer
Researchers: M.V. Efremova (Technical University of Munich, Helmholtz Center Munich, and Eindhoven University of Technology, the Netherlands); U. Wiedwald, R. Meckenstock, N. Josten, and M. Farle (University of Duisburg-Essen, Germany); F. Sigmund, S.-V. Bodea, L.N. Panzl, and G.G. Westmeyer (Technical University of Munich and Helmholtz Center Munich); H. Ohldag (ALS, Stanford University, and UC Santa Cruz); T. Feggeler (ALS and UC Berkeley); J. Francke and R. Lavrijsen (Eindhoven University of Technology, the Netherlands); I. Beer and N.P. Ivleva (Technical University of Munich); I.B. Alieva (Lomonosov Moscow State University); A.S. Garanina (National University of Science and Technology, Russian Federation); A.S. Semkina (Russian National Research Medical University); F. Curdt and M. Winklhofer (Carl von Ossietzky University of Oldenburg, Germany); S. Wintz (Max Planck Institute for Intelligent Systems, Germany); and M.A. Abakumov (National University of Science and Technology and Russian National Research Medical University).
Funding: Helmholtz Association, Russian Science Foundation, Alexander von Humboldt Foundation, Federation of European Biochemical Societies, Joachim Herz Foundation, European Union Horizon 2020, European Research Council, and German Science Foundation. Operation of the ALS is supported by the US Department of Energy, Office of Science, Basic Energy Sciences program.
Publication: M.V. Efremova, U. Wiedwald, F. Sigmund, S.-V. Bodea, H. Ohldag, T. Feggeler, R. Meckenstock, L.N. Panzl, J. Francke, I. Beer, N.P. Ivleva, I.B. Alieva, A.S. Garanina, A.S. Semkina, F. Curdt, N. Josten, S. Wintz, M. Farle, R. Lavrijsen, M.A. Abakumov, M. Winklhofer, and G.G. Westmeyer, “Genetically controlled iron oxide biomineralization in encapsulin nanocompartments for magnetic manipulation of a mammalian cell line,” Adv. Funct. Mater. 35, 2418013 (2025), doi:10.1002/adfm.202418013.
ALS SCIENCE HIGHLIGHT #521