Bioengineers seek to tweak naturally occurring enzymes in order to modify the chemicals they produce. For example, bacterial enzymes catalyze the production of nylon precursors, but if a certain biochemical “reactive handle” could be introduced into the enzymatic machinery, it would provide a versatile focal point for making alterations to the polymer, such as increasing its strength or making it flame retardant.
The reactive handle in this work is a functional group of molecules that attaches to the end of a carbon chain—a terminal alkene. In particular, this study focused on an enzyme, TcsD, that produces terminal alkenes. To learn more about how it accomplishes this, researchers performed protein crystallography studies on TcsD at ALS Beamline 5.0.2, part of the Berkeley Center for Structural Biology (BCSB).
The results revealed shifts in the structure of TcsD compared to enzymes that produce internal alkenes, which are more common than terminal alkenes, but less useful for bioengineering. The shifts surprisingly occur not only at the enzyme’s active site, but also at more distant portions of the structure.
Combined with biochemical analyses, the results allowed the researchers to the identify the amino-acid sequences that encode TcsD function. By looking for those sequences in other distantly related enzymes, the researchers found additional natural enzymes that can likely generate terminal alkenes as well.
With this new understanding of how terminal alkenes are made in nature, the researchers hope to tweak bacteria such as E. coli to produce these reactive handles as a first step toward biosynthesizing diverse polymers with tailored properties.
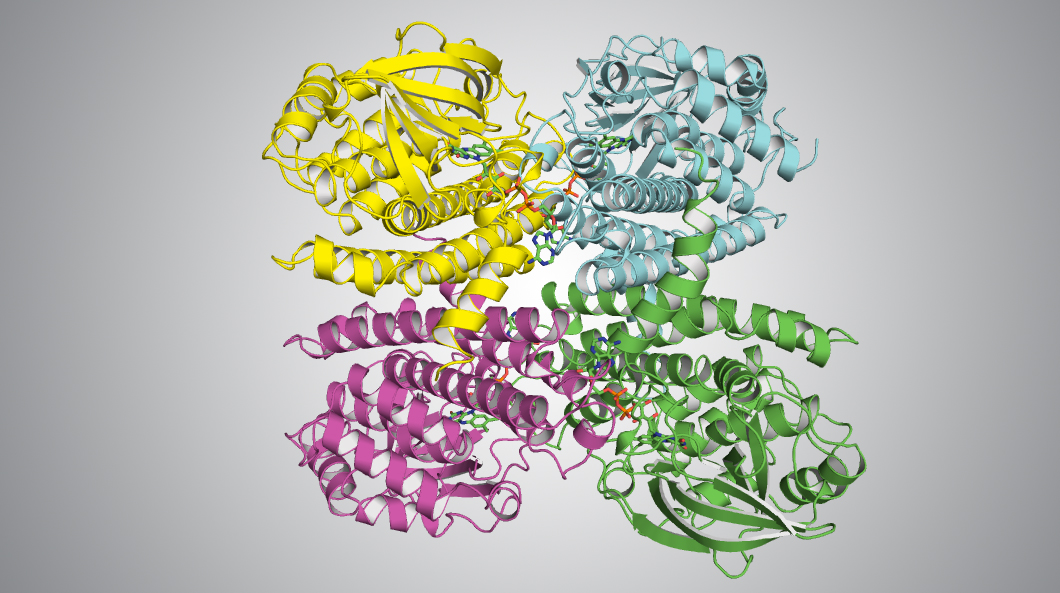
J.M. Blake-Hedges, J.H. Pereira, P. Cruz-Morales, M.G. Thompson, J.F. Barajas, J. Chen, R.N. Krishna, L.J.G. Chan, D. Nimlos, C. Alonso-Martinez, E.E.K. Baidoo, Y. Chen, J.W. Gin, L. Katz, C.J. Petzold, P.D. Adams, and J.D. Keasling, “Structural Mechanism of Regioselectivity in an Unusual Bacterial Acyl-CoA Dehydrogenase,” JACS 142, 835 (2020), doi: doi.org/10.1021/jacs.9b09187.