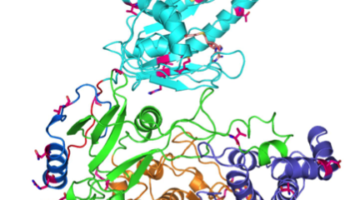
Zika virus is a mosquito-borne infectious disease linked to certain birth defects in infants. Scientists have mapped a key viral protein called NS5, which contains two enzymes: one reduces the body’s ability to mount an immune response against infection and the other helps start the genetic replication process. Read more »
ALS Work Using Protein Crystallography
Protein crystallography is used for determining the molecular structure of proteins. Crystallized protein molecules cause a beam of incident x-rays to scatter in many directions, with constructive and destructive interference generating a diffraction pattern. By analyzing these patterns, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal and thus determine the protein's structure.
Could This Enzyme Help Turn Biofuel Waste into Something Useful?
A protein used by common soil bacteria is providing new clues in the effort to convert aryl compounds, a common waste product from industrial and agricultural practices, into something of value. This Joint BioEnergy Institute (JBEI) study, which involved ALS Beamline 8.2.2, targets LigM for its role in breaking down aromatic pollutants. Read more »
NCAA Drives Formation of Designed Proteins
A noncanonical amino-acid (NCAA) complex has been found to drive the self-assembly of a computationally designed protein. Bpy-ala, which is “noncanonical” because it’s not among the 20 amino acids that occur naturally, has useful properties that could be used to generate novel photoactive proteins. Read more »
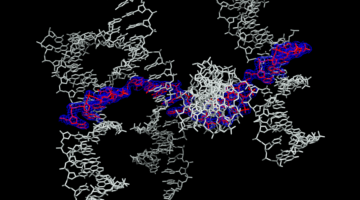
Self-Assembly of a Programmable DNA Lattice
The use of DNA for nanotechnology has gained interest because it is a highly “programmable” polymer with “sticky ends,” allowing the self-assembly of molecular scaffolds for other proteins and molecules. Their high-resolution structures will help map new routes toward the rational design of self-assembling 3D DNA crystals. Read more »
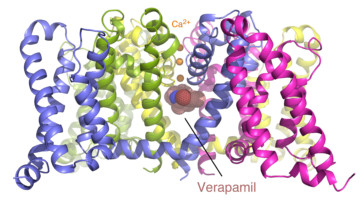
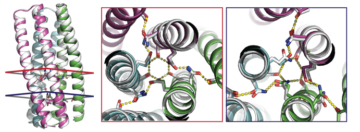
Two Basic Mechanisms of Cardiovascular Drugs
The structures of proteins controlling calcium-ion transport through cell membranes have been revealed, bound to two drugs known as calcium channel blockers. The discovery might accelerate the development of safer and more effective drugs for treating cardiovascular disorders such as high blood pressure, chest pain, and irregular heartbeat. Read more »![]()
![]()
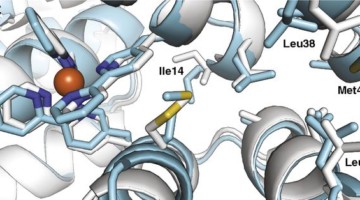
Evolutionary drivers of thermoadaptation in enzyme catalysis
With early life likely to have existed in a hot environment, enzymes had to cope with an inherent drop in catalytic speed caused by lowered temperature. Here, researchers characterize the molecular mechanisms underlying thermoadaptation of enzyme catalysis in adenylate kinase using ancestral sequence reconstruction spanning 3 billion years of evolution. Read more »
Molecular Switch Triggers Bacterial Pathogenicity
Using an array of high-powered x-ray imaging techniques at the ALS, scientists have revealed for the first time the molecular steps that turn on bacteria’s pathogenic genes. The study could open up new avenues in the development of drugs to prevent or treat bacterial infection. Read more »![]()
![]()
ALS Beamstop Device an R&D 100 Finalist
A beamstop device recently developed at the ALS has successfully combined two essential crystallographic functions–capturing the damaging portion of the beam while simultaneously monitoring its intensity–into a single miniaturized package. The technology has been licensed and launched commercially and is also a finalist for an R&D 100 Award. Read more »![]()
Validation of Novel Proteins Inspired by Nature
Designed proteins containing hydrogen-bonding modules have been validated by crystallography and SAXS. The ability to design synthetic molecules that combine the specificity of DNA-like binding with protein function opens up huge opportunities for the fields of synthetic biology and materials science. Read more »
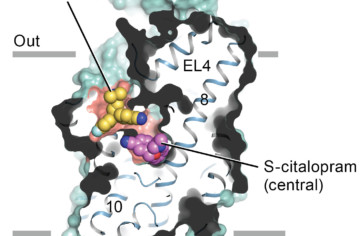
How Antidepressants Block Serotonin Transport
Malfunctions in the complex protein “machinery” of serotonin transport can result in depression, obsessive-compulsive disorder, aggression, anxiety, and Parkinson’s disease. Now, researchers have obtained x-ray crystallographic structures of the difficult-to-crystallize human serotonin transporter bound to two commonly prescribed antidepressant drug molecules. Read more »![]()
![]()
- « Previous Page
- 1
- …
- 10
- 11
- 12
- 13
- 14
- 15
- Next Page »