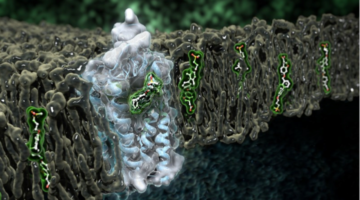
Type 2 diabetes mellitus (T2DM), characterized by abnormally high blood glucose levels, affects hundreds of millions of people worldwide. In the pursuit to better treat this disease, the human receptor protein GPR40 has been identified by pharmaceutical company Takeda as a potential new drug target.
ALS Work Using Protein Crystallography
Protein crystallography is used for determining the molecular structure of proteins. Crystallized protein molecules cause a beam of incident x-rays to scatter in many directions, with constructive and destructive interference generating a diffraction pattern. By analyzing these patterns, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal and thus determine the protein's structure.
Brain Receptor Structures Key to Future Therapeutics
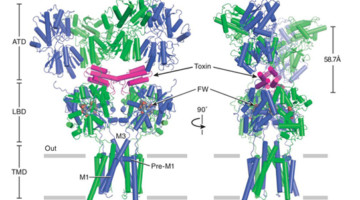
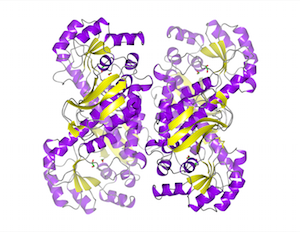
Neurotransmitter receptor proteins are critical to learning and memory. Mutations are associated with neurological and neuropsychiatric conditions including Alzheimer’s, epilepsy, and autism. Structures of two such receptors, solved by x-ray crystallography, provide a blueprint for the development of therapeutics. Read more »![]()
![]()
Designer Proteins Target Epstein-Barr-Virus-Associated Cancer
Researchers used new protein design approaches to develop a potential inhibitor of Epstein-Barr-Virus-associated cancer. The study shows not just how to help defeat the virus, but also opens up a whole new way to design proteins against viruses and ultimately, cancer. Read more »![]()
![]()
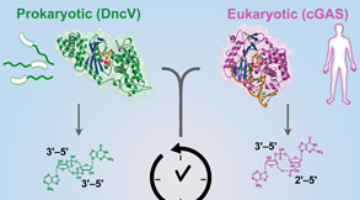
A New Link Between Human and Bacterial Signaling Machinery
Researchers showed that a bacterial signaling protein critical for pathogenesis in Vibrio cholerae is actually a homolog of the human enzyme, cGAS, which detects invading DNA. These results reveal a surprising evolutionary connection between bacterial signaling and human innate immunity. Read more »
Caribou Biosciences Has Roots at the ALS
When Rachel Haurwitz joined UC Berkeley biology professor Jennifer Doudna’s lab in 2007 as a graduate student, little did the two women know that the interesting bacterial immune system they were studying would be the subject of news headlines and the basis for a biotech startup just a few years later.
Validating Computer-Designed Proteins for Vaccines
Computationally designed proteins that accurately mimic key viral structures can help produce better vaccines. The resulting protein structures, validated at the ALS, encourage the further development of this strategy for a variety of vaccine targets, including HIV and influenza. Read more »![]()
![]()
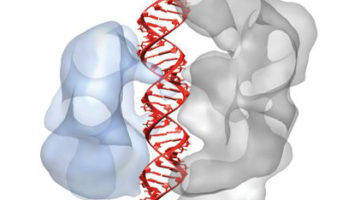
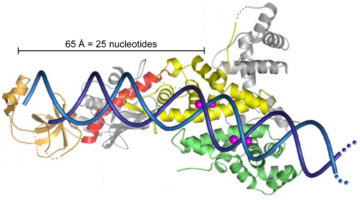
Intriguing DNA Editor Has a Structural Trigger
The molecular structures of two proteins from a family of genome-editing enzymes reveal how they target and cleave DNA. The results point the way to the rational design of new and improved versions of the enzymes for basic research and genetic engineering. Read more »![]()
![]()
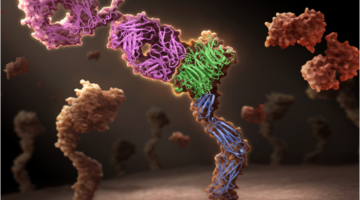
Genentech Uses ALS Crystallography for Therapeutic Antibody Research
Genentech has developed a unique one-armed antibody, onartuzumab, which is now in late-stage clinical trials in multiple cancer types. The company used crystal structures obtained at ALS Beamline 5.0.2 to demonstrate the mechanism of action of this unique potentially therapeutic antibody. Read more »![]()
![]()
Solving Structures with Collaborative Crystallography
The Berkeley Center for Structural Biology’s Collaborative Crystallography (CC) program is making major advancements in solving protein structures, especially for users involved in high-throughput projects. The CC program is an NIH-funded, peer-reviewed service that allows external users to apply for both beam time and the support of a crystallographer to perform experiments and subsequent data analyses. Read more »
First Detailed Look at RNA Dicer
Scientists have gotten their first detailed look at the molecular structure of an enzyme that Nature has been using for eons to help silence unwanted genetic messages: Dicer, an enzyme that plays a critical role in a process known as RNA interference.
Read more »![]()
![]()