Researchers describe potent small-molecule inhibitors that are neuroprotective in preclinical models of nerve injury and disease. The cover depicts the destruction of an axon by the enzyme SARM1, shown disproportionately large to convey its catastrophic role in driving degeneration once it is activated upon injury. Read more »
ALS Work Using Protein Crystallography
Protein crystallography is used for determining the molecular structure of proteins. Crystallized protein molecules cause a beam of incident x-rays to scatter in many directions, with constructive and destructive interference generating a diffraction pattern. By analyzing these patterns, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal and thus determine the protein's structure.
Protein Assemblies Show Surprising Variability
Protein-structure studies performed in part at the ALS helped researchers discover that the protein assemblies in a key carbon-cycling enzyme can rearrange with surprising ease. The findings raise the prospect of genetically tuning the protein in agricultural plant species to produce more productive and resource-efficient crops. Read more »![]()
![]()
Chemical acylation of an acquired serine suppresses oncogenic signaling of K-Ras(G12S)
Small-molecule targeting of particular KRAS mutations offer promise for cancer therapy. The cover depicts a small-molecule ligand (red) inhibiting the oncogenic mutant protein K-Ras(G12S) (cyan) by forming a covalent ester adduct at the mutant serine. Read more »
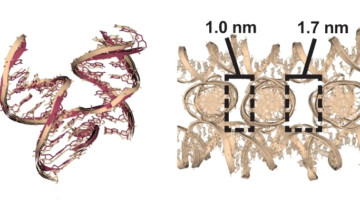
An Expanded Set of DNA Building Blocks for 3D Lattices
Researchers studied 36 DNA-based molecular junctions and discovered factors that yield superior self-assembled 3D lattice structures. The work expands the set of building blocks for lattices that can scaffold molecules into regular arrays, from proteins for structure studies to nanoparticles for nano-antennas and single-particle sensors. Read more »![]()
![]()
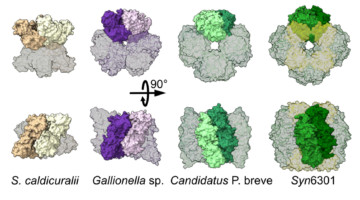
Protein Structures Aren’t Set in Stone
A group of researchers studying the world’s most abundant protein, an enzyme involved in photosynthesis called rubisco, showed how evolution can lead to a surprising diversity of molecular assemblies that all accomplish the same task. The findings reveal the possibility that many of the proteins we thought we knew actually exist in other, unknown shapes. Read more »
Deep-Learning AI Program Accurately Predicts Key Rotavirus Protein Fold
Rotaviruses are the major causative agents of gastroenteritis worldwide. Attempts to design vaccines are complicated by the rotaviruses’ enormous genetic and immunological diversity. At the ALS, researchers validated the novel structure of a key rotavirus protein, predicted using AlphaFold2, a deep-learning artificial-intelligence program. Read more »
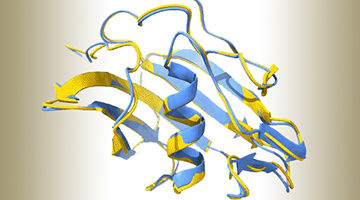
Molecular Switch Triggers Changes in Plant Structure
Using x-ray crystallography, biochemistry, and plant genetics, researchers identified a molecular switch that triggers modifications to plant structure in response to environmental conditions. A greater understanding of this adaptive process will help scientists optimize plants for efficient nutrient uptake and resistance to parasitic species. Read more »![]()
![]()
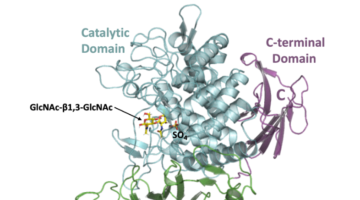
Bacterial Enzyme Produces Biodegradable Polymer
Researchers discovered a bacterial enzyme that synthesizes a biopolymer whose repeating units are linked together in way that had not been previously observed. The new polymer is biodegradable and may be biocompatible, with potential for applications ranging from medical therapeutics to eco-friendly plastic alternatives. Read more »![]()
![]()
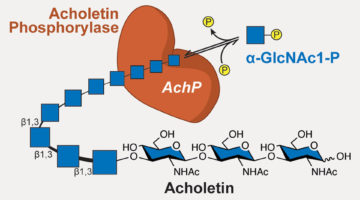
Newly Discovered Bacterial Enzyme Produces Useful Biopolymer
Researchers identified a bacterial enzyme that produces a novel biopolymer. The polymer, dubbed acholetin, is a chain of sugar molecules known as a polysaccharide. Acholetin is similar in structure to chitin, the major component of insect exoskeletons, and holds promise as a useful biomaterial because of its biodegradability and biocompatibility. Read more »
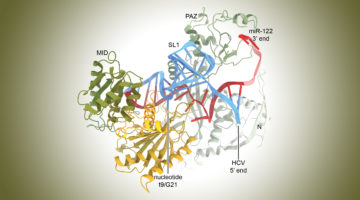
Molecular Hijacking of a MicroRNA by the Hepatitis C Virus
The hepatitis C virus (HCV), which attacks the liver, is known to repurpose host-cell components known as microRNAs—short RNA strands that act to silence gene expression. Now, the molecular structure of an HCV site bound to a microRNA complex revealed how their interactions shield the virus from the host cell’s protective response. Read more »
- « Previous Page
- 1
- 2
- 3
- 4
- 5
- …
- 15
- Next Page »