Anaerobic SEC-MALS-SAXS at the SIBYLS beamline probes the conformational states behind electron bifurcation in the Thermotoga maritima EtfABCX, revealing insights on mechanisms at the thermodynamic limits of life. Shown are the bifurcation- and electron-conducting-like states experimentally observed for the first time in solution. Read more »
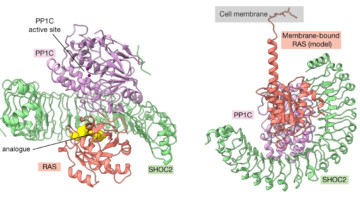
Structures Signal Fresh Targets for Anticancer Drugs
Researchers from Genentech used a suite of methods, including small-angle x-ray scattering, to learn how an assembly of three proteins works together to transmit signals for cell division. The work reveals new targets for the development of drugs that fight certain types of cancer, including lung, colorectal, and pancreatic cancer. Read more »![]()
![]()
Protein Structures Aren’t Set in Stone
A group of researchers studying the world’s most abundant protein, an enzyme involved in photosynthesis called rubisco, showed how evolution can lead to a surprising diversity of molecular assemblies that all accomplish the same task. The findings reveal the possibility that many of the proteins we thought we knew actually exist in other, unknown shapes. Read more »
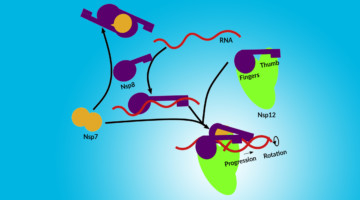
Assembly of the SARS-CoV-2 Replication Mechanism
Using a multimodal approach that included x-ray scattering at the ALS, researchers determined how components of the SARS-CoV-2 replication mechanism fit together. A better understanding of how this protein complex works provides insight into potential structural or functional weak spots to exploit for drug development. Read more »![]()
![]()
Deconstructing the Infectious Machinery of the SARS-CoV-2 Virus
Scientists collaborated to model the complex protein responsible for SARS-CoV-2 replication, revealing its potential weak spots for drug development. The investigation hinged on data collected from many advanced imaging techniques, including small-angle x-ray scattering (SAXS), crystallography, and small-angle neutron scattering (SANS). Read more »
Study Finds ‘Missing Link’ in the Evolutionary History of Carbon-Fixing Protein Rubisco
Scientists discovered an ancient form of rubisco, the most abundant enzyme on Earth and critical to life as we know it. Found in previously unknown environmental microbes, the newly identified rubisco provides insight into the evolution of the photosynthetic organisms that underlie the planet’s food chains. Read more »
Providing New Technologies for Vaccine Development
Antigens can sometimes be attached to a protein scaffold to mimic the shape of a virus and elicit a stronger immune response. Scientists developed a method to design such proteins, and ALS data helped to visualize the atomic structure and determine the dynamics of the designed scaffolds. Read more »
This Enigmatic Protein Sculpts DNA to Repair Harmful Damage
Scientists have discovered that a DNA-repairing protein performs its functions by first marking and then further breaking damaged DNA. The surprising findings have provided much-needed insight into how DNA repair works in healthy human cells, as well as how different mutations can translate into different diseases and cancer. Read more »
Assembly Lines for Designer Bioactive Compounds
Researchers successfully bioengineered changes to a molecular “assembly line” for bioactive compounds, based in part on insights gained from small-angle x-ray scattering at the ALS. The ability to re-engineer these assembly lines could improve their performance and facilitate the synthesis of new medically useful compounds. Read more »![]()
![]()
X-Ray Technology Sheds New Light on Antibiotic Synthesis
Atomic-scale structural analyses performed at the ALS are helping scientists understand the inner workings of the enzyme “assembly lines” that microbes use to produce an important class of compounds, many of which have uses as antibiotics, antifungals, and immunosuppressants. Read more »