SCIENTIFIC ACHIEVEMENT
Scientists identified a mechanism for the formation of soot involving a series rapid chemical reactions rather than the typical condensation of particles from gas.
SIGNIFICANCE AND IMPACT
The results are critical to developing methods for controlling emissions responsible for millions of deaths annually, severe degradation of air quality, and enhanced global warming.
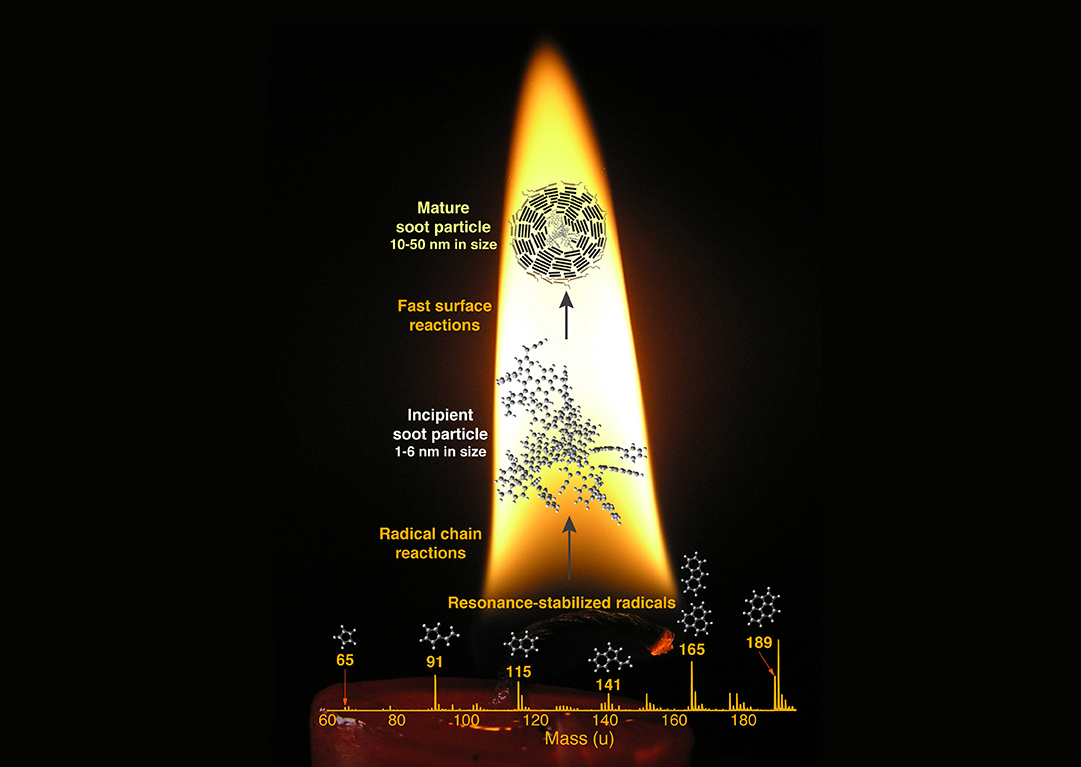
The mystery of soot formation
Soot is responsible for millions of deaths annually and contributes substantially to climate change as well as indoor and outdoor air pollution. Understanding the fundamental chemistry behind soot formation is the first step in being able to reduce soot emissions to the atmosphere.
Scientists have been trying for decades to explain “soot inception,” the initial steps in soot formation in which gas-phase hydrocarbon molecules transition into condensed-phase incipient particles at high temperature. One hypothesized route to inception is via a thermodynamically driven nucleation process. At soot-formation temperatures of 1400–1700 K, however, the condensed phase of the hydrocarbons in the flame is not thermodynamically stable. A mechanism for the formation of less volatile, condensable species has thus far not been identified. The alternative route is via chemical reactions that covalently bond or cluster available hydrocarbons. Until now, however, proposed chemical mechanisms have been too slow to explain the observed rapid rate of soot formation.
Identification of the smoking gun
The key evidence for solving the mystery of soot formation was provided by measurements made at ALS Beamline 9.0 using aerosol mass spectrometry (AMS) with vacuum-ultraviolet (VUV) photoionization (watch video here). Tunable synchrotron VUV light allows “soft” ionization, which minimizes the molecular fragmentation of the large hydrocarbon species likely to be involved in soot-forming chemical reactions. Measurements were made on incipient soot particles extracted from a variety of flames. The VUV-AMS data revealed a series of peaks corresponding to what’s known as resonance-stabilized radicals (RSRs). Like other radicals, RSRs have unpaired electrons that make them reactive, but these unpaired electrons are somewhat stabilized by double bonds in the molecules, making them less reactive. RSR peaks were observed in every flame investigated, leading to the realization that they may be important precursors to soot inception.
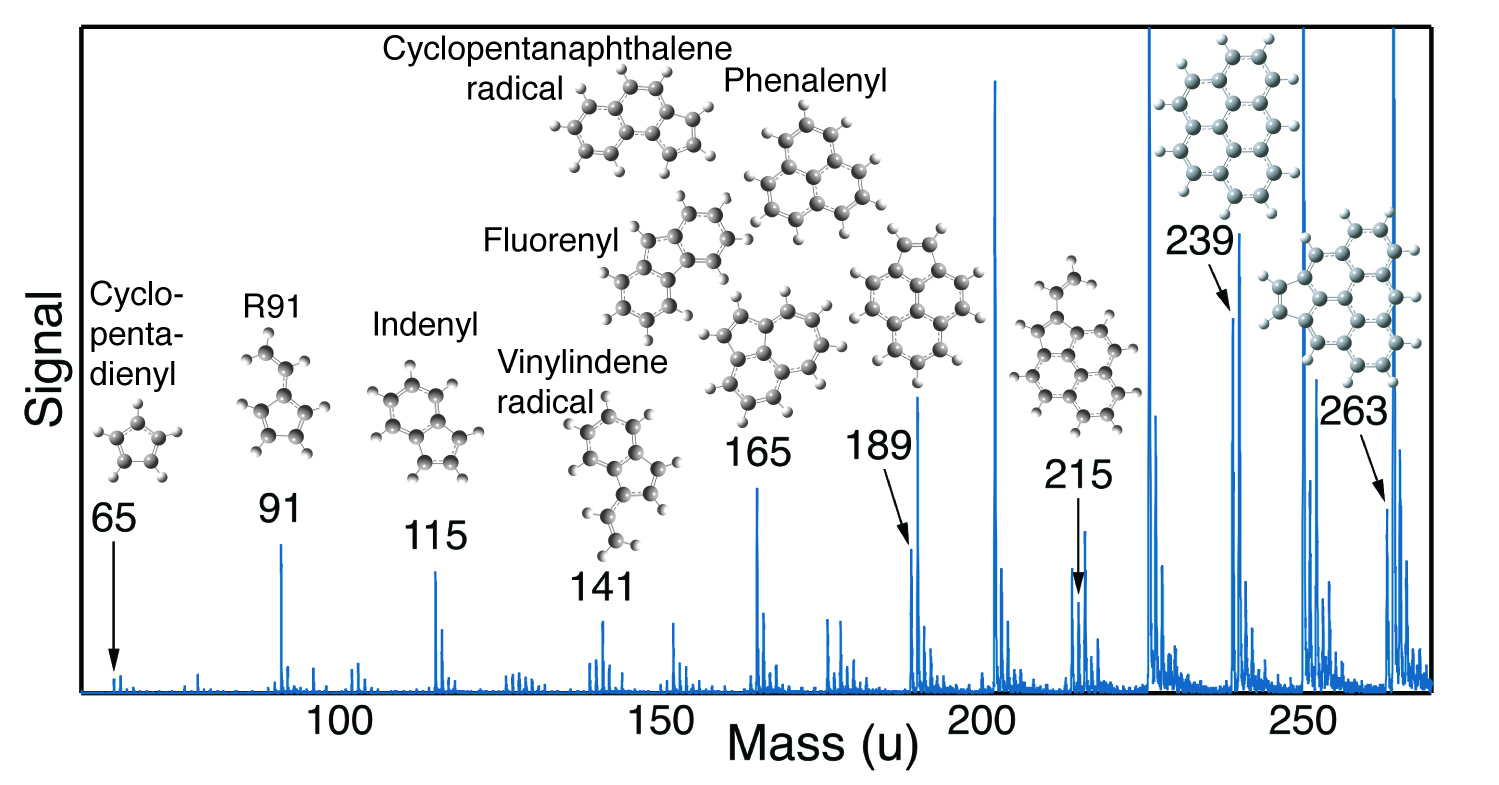
The theory of the case
The experimental evidence for RSRs was only part of the solution to soot formation; the final solution required interpretation of the evidence. Quantum mechanical calculations demonstrated that the sequence of RSRs observed could be readily formed by repeated addition of acetylene or vinyl radicals. The ground-breaking part of the mechanism, however, was the discovery that any of these RSRs could initiate the formation of covalently bound hydrocarbon clusters through a radical chain reaction. The reactions of RSRs with other RSRs and non-radical species generate products that are not radicals, but simply losing a hydrogen atom from these products regenerates an RSR. Radical chain reactions are notorious for being very fast. Many researchers had looked at RSR reactions but had focused on how to generate stable polycyclic aromatic hydrocarbons via such reactions. In other words, they had focused on chain-reaction termination steps, rather than chain-reaction propagation steps.
Final verdict needs predictive models
Now that there is a proposed mechanism for particle inception that does not violate the laws of thermodynamics, the next step is to calculate rate constants. With these rate constants, this mechanism can be integrated into models for soot formation in flames and engines. The search also continues for other mechanisms that could explain soot formation and may be more important under other conditions, such as at the high pressure relevant to engine combustion.

Contacts: K. Olof Johansson and Hope Michaelson
Researchers: K.O. Johansson, P.E. Schrader, and H.A. Michelsen (Sandia National Laboratories, Livermore); K.R. Wilson (Berkeley Lab); and M.P. Head-Gordon (University of California, Berkeley, and Berkeley Lab).
Funding: U.S. Department of Energy, Office of Science, Basic Energy Sciences Program (DOE BES). Operation of the ALS is supported by DOE BES.
Publication: K.O. Johansson, M.P. Head-Gordon, P.E. Schrader, K.R. Wilson, and H.A. Michelsen, “Resonance-stabilized hydrocarbon-radical chain reactions may explain soot inception and growth,” Science 361, 997 (2018), doi:10.1126/science.aat3417.
ALS SCIENCE HIGHLIGHT #386