SCIENTIFIC ACHIEVEMENT
Using the Advanced Light Source (ALS), researchers tracked the movement of the platinum nanoparticles that catalyze reactions in polymer electrolyte fuel cells (PEFCs) and correlated this movement with nanoparticle degradation.
SIGNIFICANCE AND IMPACT
The results yielded solutions that can immediately reduce platinum waste in emission-free heavy-duty fuel-cell vehicles.
Power for the long haul
Polymer electrolyte fuel cells (PEFCs) that run on green hydrogen are an excellent option for powering heavy-duty vehicles that produce zero greenhouse-gas emissions. This type of fuel cell has the potential to extend driving range without incurring additional weight penalties. However, PEFCs use platinum as a catalyst to drive a key chemical reaction. The fast degradation of this precious metal and its high cost currently prevent the widespread commercialization of this otherwise promising technology.
Here, a study led by scientists from UC Irvine and Bosch Research sought to better understand the nanoscale mechanisms by which platinum degrades during PEFC operation. The results point the way to improvements in PEFC fabrication that can significantly extend the lifetime and efficiency of PEFCs in heavy-duty vehicles, reduce their cost, improve air quality, and fight climate change.
Studies of fuel-cell longevity
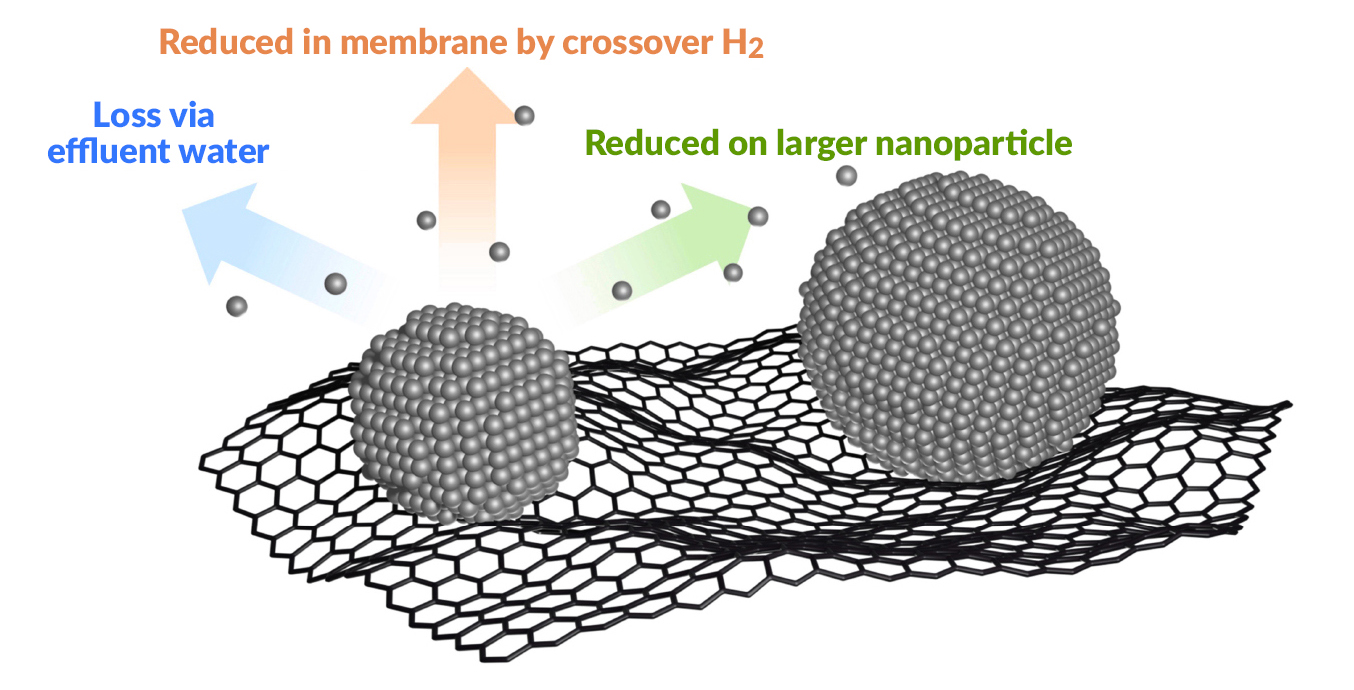
Small, well-dispersed platinum nanoparticles increase the electrochemically active surface area of PEFCs. However, repeated cycling over the lifetime of a heavy-duty vehicle results in platinum dissolution and coalescence. The researchers’ previous work at the ALS had suggested that platinum nanoparticle growth is driven strongly by the local humidity in the PEFC cathode catalyst layer (where hydrogen gas interacts with the catalyst), and that particle growth is more pronounced near the gas outlet.
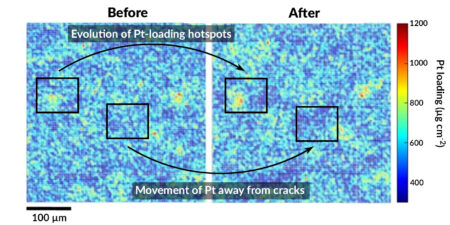
In this work, the team examined commercially available membrane electrode assemblies (MEAs) in which a polymer electrolyte membrane is sandwiched between anode and cathode catalyst layers and gas-diffusion layers on either side. Accelerated stress tests simulated conditions in heavy-duty vehicle use. Before and after the tests, the MEAs were examined using electron microscopy and lab-based fluorescence spectroscopy near the inlet and outlet of the cathode gas flow field. The results captured the in-plane movement of platinum toward local hotspots and away from cracks.
Synchrotron studies reveal correlation
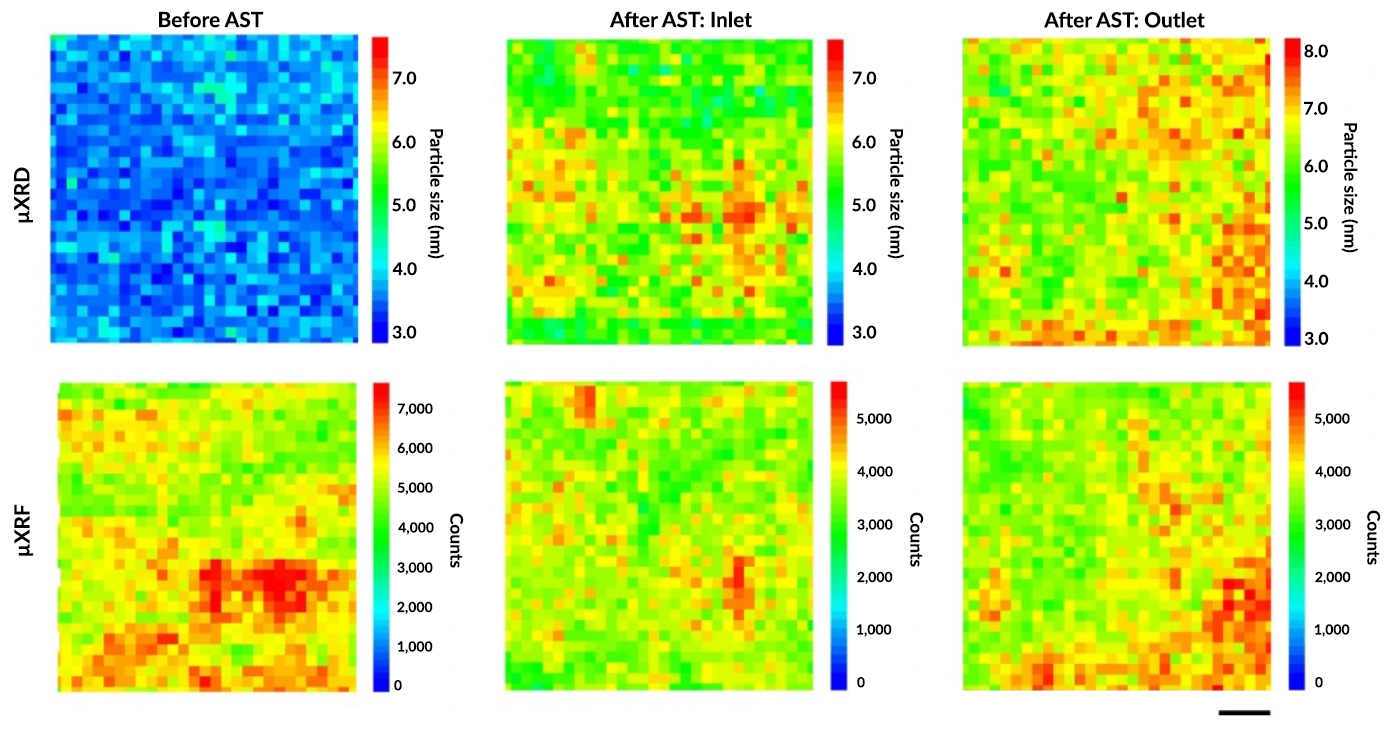
At ALS Beamline 12.3.2, the researchers used x-ray microdiffraction (µXRD) to determine particle sizes and x-ray microfluorescence (µXRF) to simultaneously map changes in platinum loading (distribution) at identical locations. The beamline’s ability to perform both experiments with micrometer spatial resolution was crucial, along with excellent beamline control and data analysis software. At Beamline 8.3.2, microcomputed tomography was also used to visualize cracks milled into the test samples.
The data revealed that, at the beginning of PEFC life, platinum nanoparticle size and distribution are independent of each other. However, a strong linear correlation develops at the end of life. Along with the other collected data, this information led the researchers to conclude that platinum degradation is strongly influenced by its initial loading in the cathode catalyst layer. Based on these findings, a possible strategy for preventing the depletion of platinum is to develop a structure with regions of larger platinum nanoparticle size and lower platinum weight percentage at locations that undergo exacerbated electrocatalyst degradation.
This research elucidates a key limiting factor in the deployment of fuel cells in heavy-duty vehicles, which is the focus of the Department of Energy’s Million Mile Fuel Cell Truck consortium. Future work could include performing these experiments operando, to understand exactly when, where, and how the changes in platinum nanoparticle size take place.
Contacts: Lei Cheng and Iryna Zenyuk
Researchers: K. Khedekar, P. Atanassov, and I.V. Zenyuk (University of California, Irvine); A. Zaffora (UC Irvine and University of Palermo, Italy); M. Santamaria (University of Palermo, Italy); M. Coats and S. Pylypenko (Colorado School of Mines); J. Braaten, L. Cheng, and C. Johnston (Bosch Research and Technology Center North America); and N. Tamura (ALS).
Funding: Bosch Research and Technology Center North America. Operation of the ALS is supported by the US Department of Energy, Office of Science, Basic Energy Sciences program.
Publication: K. Khedekar, A. Zaffora, M. Santamaria, M. Coats, S. Pylypenko, J. Braaten, P. Atanassov, N. Tamura, L. Cheng, C. Johnston, and I.V. Zenyuk, “Revealing in-plane movement of platinum in polymer electrolyte fuel cells after heavy-duty vehicle lifetime,” Nat. Catal. 6, 676 (2023), doi:10.1038/s41929-023-00993-6.
ALS SCIENCE HIGHLIGHT #493