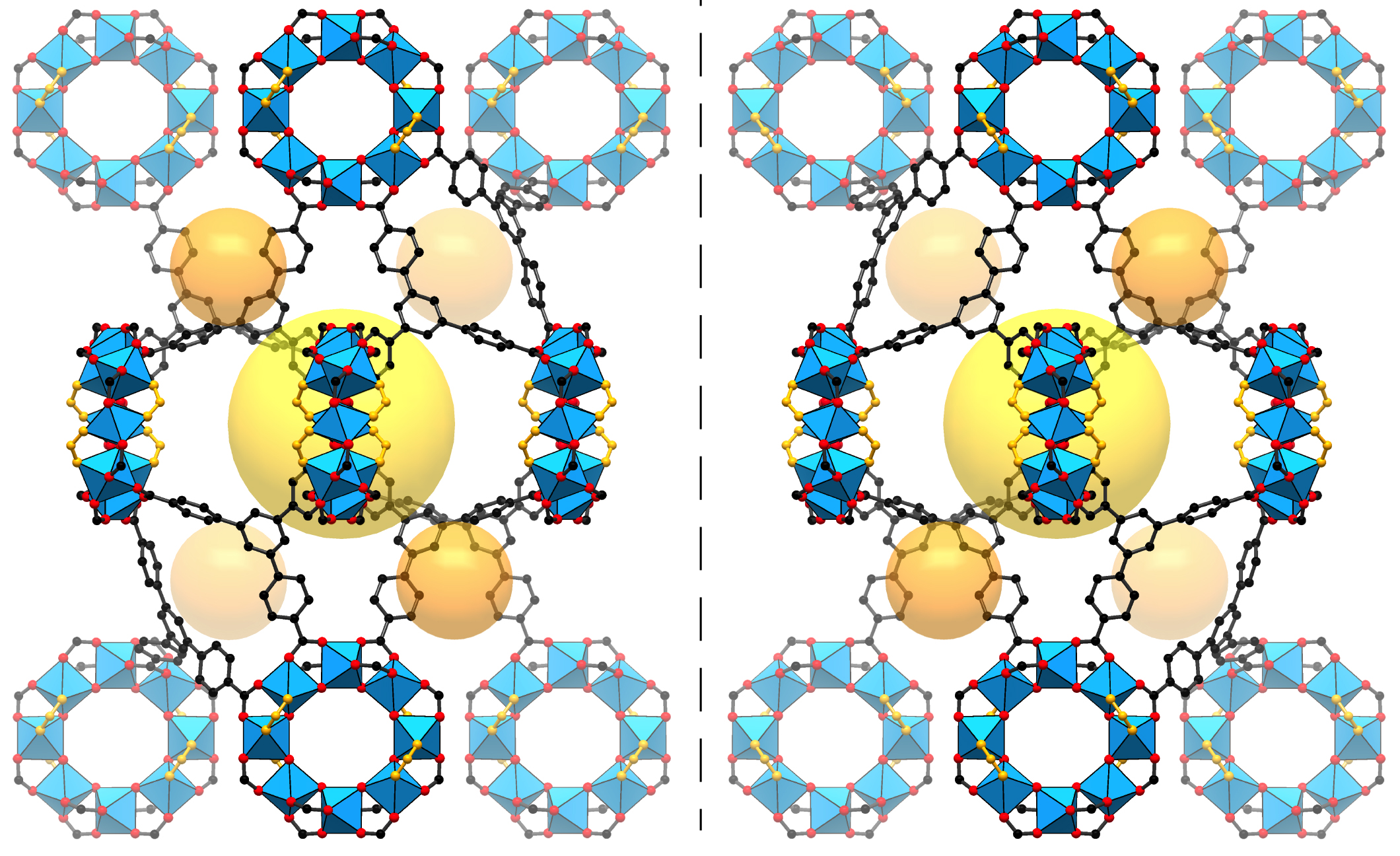
Researchers have created a sort of nanoscale display case that enables new atomic-scale views of hard-to-study chemical and biological samples. A series of different molecules were chemically bound inside sturdy structures known as metal-organic frameworks (MOFs), each measuring about 100 micrometers across. Single-crystal x-ray diffraction at the ALS was then used to determine the precise molecular structures of the samples inside the MOFs. The work could help to reveal new structural details for a range of challenging molecules—including complex chemical compounds and potentially new drugs.
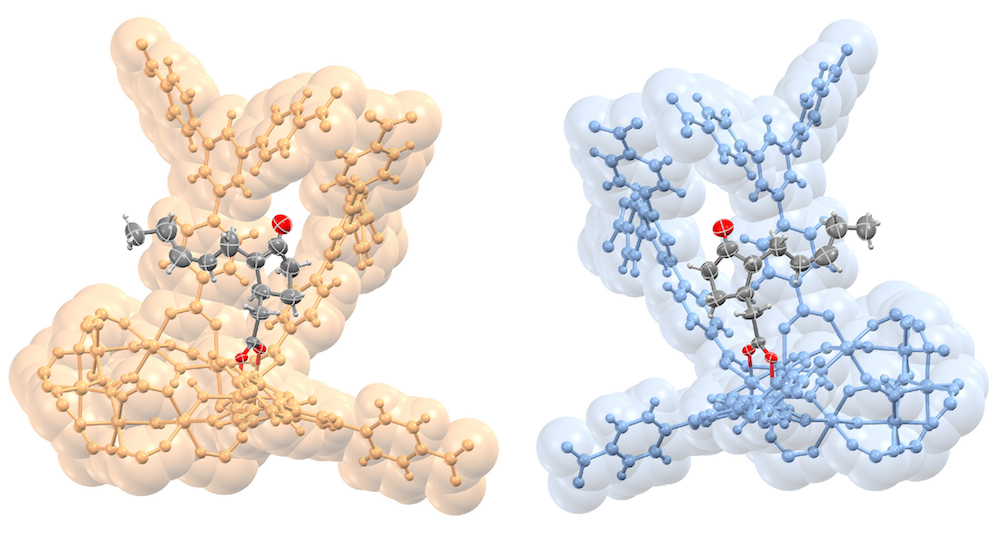
The samples studied ranged from a simple alcohol to a complex plant hormone, and the new method, dubbed “CAL” for covalent alignment (the molecules form covalent bonds in the MOFs), enables researchers to determine the complete structure of a molecule from a single MOF crystal that contains the sample molecules in its pores.
The MOFs in the study, which were identical and easy to manufacture in large numbers, provided a backbone that held the sample molecules still for the x-ray experiments—the molecules otherwise can be wobbly and difficult to stabilize. The researchers prepared the samples by dipping the MOFs into solutions containing different molecular mixes and then heating them until they crystallized.
The MOFs also possess a particular chirality (left- or right-handedness) that selectively binds with molecular samples of similar chirality. The difference in a molecule’s chirality is particularly important for pharmaceuticals, as it can mean the difference between a medicine and a poison. Hard-to-study proteins, such as those important for drug development, are high-priority targets for the new technique. Some such molecules are difficult to form into crystals, and the process of crystallizing a single molecule can in some cases involve years of effort and expense.
The researchers determined the atomic structure of the MOFs and the bound molecules using single-crystal x-ray diffraction at ALS Beamline 11.3.1, and they also studied the MOFs using nuclear magnetic resonance (NMR) at Berkeley Lab’s Molecular Foundry. In all, the researchers studied 16 different molecules bound inside the MOF pores, representing a range of functionality, flexibility, and complexity. These included a plant hormone called jasmonic acid whose chiral structure had never been directly determined before, other plant hormones known as gibberellins, methanol, and other acids and alcohols.
Importantly, the MOFs in this study did not appear to distort the natural, intact structure of the molecules. The researchers say it is possible to determine the complete 3D structure of a molecule even if the samples only fill about 30 percent of a MOF’s pores. In addition, the metals in the MOF framework itself can actually serve to enhance the quality of the x-ray images: in one case the technique allowed researchers to distinguish between two nearly identical plant hormones based on the difference in a single atomic bond.
The researchers could see structural details down to hundredths of a nanometer—less than the diameter of some atoms. With such precision, they could distinguish whether a particular atom was a carbon atom or something else. Once a molecule is bound in the MOF, the researchers found that they could learn the absolute structure very precisely since the chirality of the MOF serves as a reference during the structure refinement.
In future experiments, different types of MOFs, with different pore sizes, could be tested to find out which ones work best with different types of samples. The researchers are also interested in solving the structures of molecules that have never been crystallized before, showing not only their atomic arrangements, but also their chirality, which is of particular interest to pharmaceutical companies.
Contact: Omar Yaghi
Research conducted by: S. Lee and E.A. Kapustin (UC Berkeley, Berkeley Lab, Kavli Energy NanoSciences Institute, and Berkeley Global Science Institute) and O.M. Yaghi (UC Berkeley, Berkeley Lab, Kavli Energy NanoSciences Institute, Berkeley Global Science Institute, and King Abdulaziz City for Science and Technology, Saudi Arabia).
Research funding: This work was supported by BASF SE (Germany) and King Abdulaziz City for Science and Technology (Saudi Arabia). Operation of the ALS is supported by the U.S. Department of Energy, Office of Basic Energy Sciences.
Publication about this research: S. Lee, E.A. Kapustin, and O.M. Yaghi, “Coordinative alignment of molecules in chiral metal-organic frameworks,” Science 353, 808 (2016). doi:10.1126/science.aaf9135
ALS SCIENCE HIGHLIGHT #342