SCIENTIFIC ACHIEVEMENT
Scientists formulated and characterized a graphene-based electrocatalyst that potentially makes the production of hydrogen peroxide more selective, efficient, and cost effective.
SIGNIFICANCE AND IMPACT
Hydrogen peroxide is an important commodity chemical with a growing demand in many areas, including the electronics industry, wastewater treatment, and paper recycling.
It whitens, brightens, and disinfects!
Hydrogen peroxide (H2O2) is a common household chemical, well known for its effectiveness at whitening and disinfecting. It’s also a valuable commodity chemical used to etch circuit boards, treat wastewater, and bleach paper and pulp—a market expected to grow as demand for recycled paper products increases.
Compared to chlorine-based bleaches, hydrogen peroxide is more environmentally benign: the only degradation product of its use is water. However, it’s currently produced through a multistep chemical reaction that consumes significant amounts of energy, generates substantial waste, and requires a catalyst of palladium—a rare and expensive metal. Furthermore, the transport and storage of bulk hydrogen peroxide can be hazardous, making local, on-demand production highly desirable.
Better living through electrochemistry
Scientists seek a way to generate hydrogen peroxide electrochemically—by a much simpler process called the oxygen reduction reaction (ORR). This reaction takes oxygen from the air and combines it with water and two electrons to produce H2O2. If this reaction could be efficiently catalyzed, it could enable the disinfection of water at remote locations, or during disaster recovery, using hydrogen peroxide made from local air and water. For this work, the researchers focused on hydrogen peroxide synthesis in alkaline environments, where the reaction bath can be used directly, such as for bleaching or the treatment of acidic waste streams.
To realize the advantages of electrochemical production, however, electrodes that catalyze the ORR selectively, efficiently, and cost effectively need to be developed. Carbon, for example, is abundant and inexpensive. In graphene oxide form, it’s characterized by oxygen defects that act as catalytic centers. Because these oxygen defects are removed by “aggressive” treatment during synthesis (exposure to oxidizing agents, sulfuric acid, heat, and pressure), the researchers used a “mild” reduction process to preserve as many as possible. The challenge then was to discover which defects resulted in the highest catalytic activity and selectivity.
Defect effects detected
The researchers found that their mildly reduced graphene oxide (mrGO) showed highly selective, stable, and efficient peroxide formation under basic conditions, exceeding the performance of current state-of-the-art alkaline catalysts. They then used several laboratory-based experimental techniques (including infrared, x-ray photoelectron, and in situ Raman spectroscopies) to better understand the relationship between defect structures and catalytic properties.
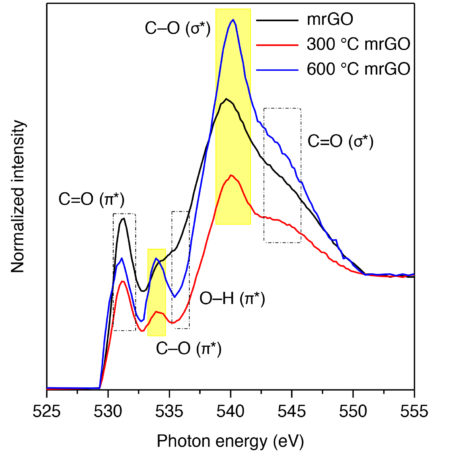
Synchrotron-based near-edge x-ray absorption fine-structure (NEXAFS) spectroscopy at ALS Beamline 8.0.1 was used to explore how annealing the samples influenced defect-site composition. Previous studies had shown that annealing substantially reduces oxygen content. The energy resolution of the beamline at the oxygen K-edge and its high photon flux are well suited to the characterization of materials with relatively low oxygen content. Surprisingly, the strongest oxygen signal came from the sample annealed at the highest temperature. This peak signified that certain types of oxygen defects (“ring ethers”), located near the edges of the graphene oxide plane, were primarily responsible for the observed catalytic activity. Data from the other spectroscopic techniques supported this conclusion as well.
The results point the way toward effective strategies for optimizing graphene oxide peformance through an easily scalable, mild thermal reduction process. Overall, the work demonstrates that carbon-based electrocatalysts hold great promise for more distributed, environmentally friendly hydrogen peroxide production.
Contact: Bryan McCloskey
Researchers: H.W. Kim, P. Yang, and B. McCloskey (UC Berkeley and Berkeley Lab); M. Ross and N. Kornienko (UC Berkeley); and L. Zhang and J.-H. Guo (ALS).
Funding: National Science Foundation, National Research Foundation of Korea, Royal Society International Newton Fellowship, and U.S. Department of Energy, Office of Science, Basic Energy Sciences Program (DOE BES). Operation of the ALS is supported by DOE BES.
Publication: H.W. Kim, M. Ross, N. Kornienko, L. Zhang, J.-H. Guo, P. Yang, and B. McCloskey, “Efficient hydrogen peroxide generation using reduced graphene oxide based oxygen reduction electrocatalysts,” Nat. Catal. 1, 282 (2018), 10.1038/s41929-018-0044-2.
ALS SCIENCE HIGHLIGHT #380