A team of scientists from the Howard Hughes Medical Institute’s Janelia Research Campus designed and validated via x-ray crystallographic studies a fluorescent protein (CaMPARI) that allows the permanent marking of active brain cells. The protein was then used to study live changes via fluorescence in the active nerve cells in brains of fruit flies, zebrafish, and mice.
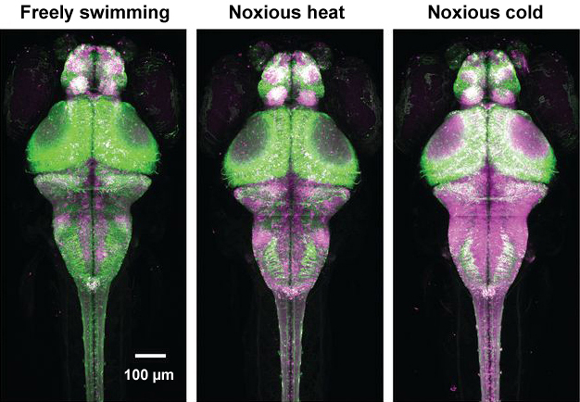
In theory, one can monitor the levels of calcium ions in brain cells to see which circuits are lighting up and when. But the signals are transient, so that after the activity is over (sometimes as fast as microseconds) the ion levels change again. Closely related previous studies used dyes or proteins that change color when they bind to calcium, with the color change being monitored with a microscope. This approach is an excellent step in imaging brain circuits, but a limitation is that the microscope has to be pointing at the area of activity when it changes, otherwise it will miss the event. Another limitation is that the brain being monitored has to be still, so that the microscope can focus on the area where the calcium levels are changing.
The breakthrough made by this research team was to combine two different protein “technologies.” The first technology is a protein that permanently changes color when it binds to calcium. Using this protein, neural activity (as measured by calcium concentration) can be stored in the color of the protein, like a switch that has flipped and only has to be looked at later to determine whether it is on or off. The other key aspect of the breakthrough was to ensure that the change in color only takes place when the calcium-bound protein is exposed to violet light. This meant that the scientists could shine violet light on the animal while it was engaged in a particular activity (for instance, when a fruit fly was exposed to a particular odor), and the brain circuits firing during that particular activity are then effectively captured for later study. The designer protein is called CaMPARI (calcium-modulated photoactivatable ratiometric integrator).
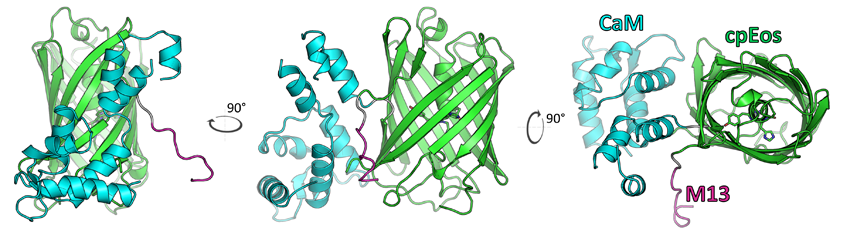
The part of CaMPARI that binds to calcium is called calmodulin, and the part that fluoresces in different colors in response to violet light is called Eos. Engineering CaMPARI required combining these two components in thousands of different variations until the product gave a high enough response to both calcium concentration and light. The researchers in this study then used it to show brain activity in zebrafish as a response to water temperature and to trace the brain-circuit response of fruit flies to different odors.
How does protein crystallography play a role in all of this? As in many cases, crystallography offers a way to visualize the underlying structure of a molecule in order to both validate a design and further improve on a design. In the case of CaMPARI, the scientists needed to know the structure of the new tool that they had designed in order to understand exactly how it worked. They brought CaMPARI to Beamline 8.2.2 at the ALS to solve its molecular structure. The results show exactly how Eos and calmodulin are connected and how the engineered mutations in each allow the combined proteins to function together as one unit.
CaMPARI is an outstanding new tool for mapping brain activity, but it could be made even more sensitive and versatile. The researchers in this study are continuing to improve it, but they are also releasing all information about CaMPARI, as well as the DNA needed to produce it and the zebrafish expressing CaMPARI, to make it available for other scientists. In this way, other scientists can not only use it, but improve on it as well. After all, the more brains working on brain tools, the better.
Contact: Eric Schreiter
Research conducted by: B.F. Fosque, Y. Sun, H. Dana, C.-T. Yang, T. Ohyama, M.R. Tadross, R. Patel, M. Zlatic, D.S. Kim, M.B. Ahrens, V. Jayaraman, L.L. Looger, and E.R. Schreiter (Howard Hughes Medical Institute).
Research funding: Howard Hughes Medical Institute. Operation of the ALS is supported by the U.S. Department of Energy, Office of Basic Energy Sciences.
Publication about this research: B.F. Fosque, Y. Sun, H. Dana, C.-T. Yang, T. Ohyama, M.R. Tadross, R. Patel, M. Zlatic, D.S. Kim, M.B. Ahrens, V. Jayaraman, L.L. Looger, and E.R. Schreiter, “Labeling of active neural circuits in vivo with designed calcium integrators,” Science 347, 755 (2015).
ALS SCIENCE HIGHLIGHT #318