SCIENTIFIC ACHIEVEMENT
Researchers integrated several approaches, such as cryogenic 3D imaging at the Advanced Light Source (ALS), to define a novel cellular pathway—involving a shared “garbage dump”—for clearing misfolded proteins from cells.
SIGNIFICANCE AND IMPACT
The pathway is a potential therapy target for age-related diseases like Alzheimer’s, Huntington’s, and Parkinson’s diseases.
Protein quality control
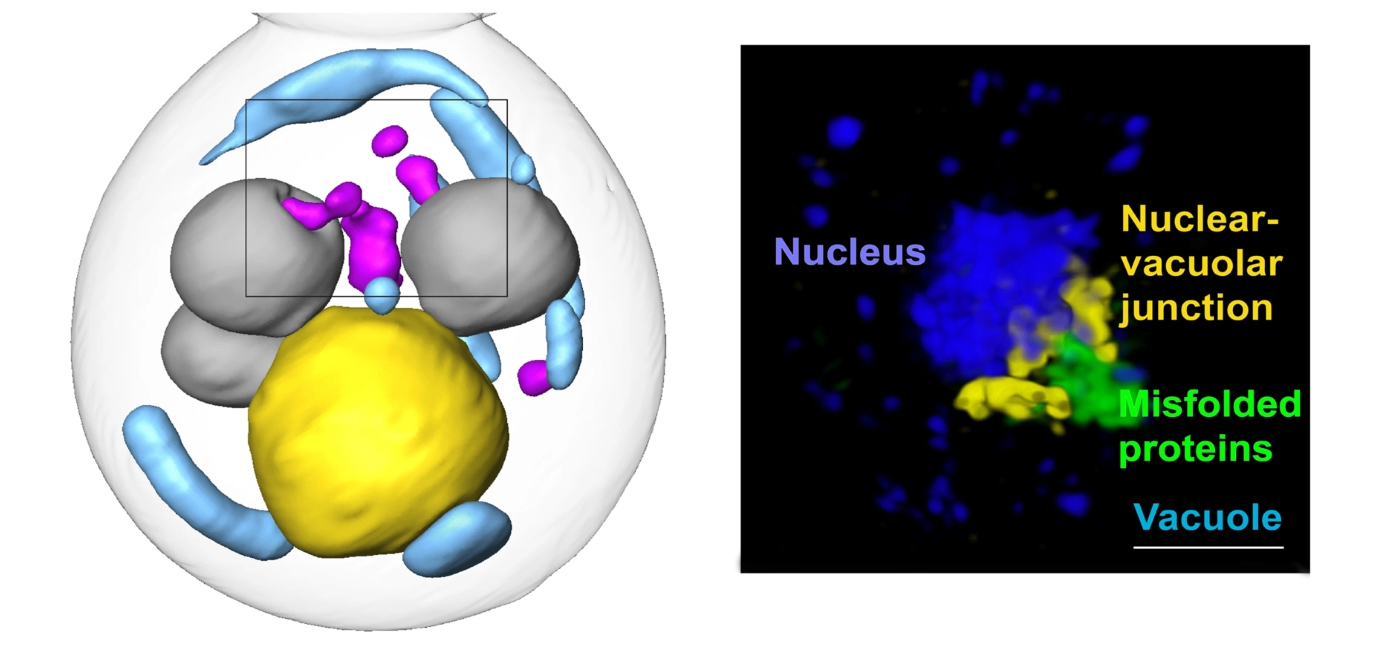
Misfolded proteins are toxic to cells. They disrupt normal functions and cause some age-related human degenerative diseases, like Alzheimer’s, Parkinson’s, and Huntington’s diseases. Cells work constantly to eliminate misfolded proteins, but these quality-control mechanisms are still poorly understood. In this study, researchers found a previously unknown cellular pathway for clearing misfolded proteins from the nucleus, a discovery that could lead to new targets for age-related disease therapies.
To find the new pathway, the researchers integrated several imaging, biochemical, and genetic approaches. For the experiments, the team restricted misfolded proteins in yeast cells to either the nucleus or the cytoplasm—the area inside the cell but outside the nucleus. The team visually followed the fate of the misfolded proteins through soft x-ray tomographic imaging at the ALS and super-resolution structured illumination microscopy (SIM), a visible-light technique that allows resolution beyond the diffraction limit.
A shared “garbage dump”
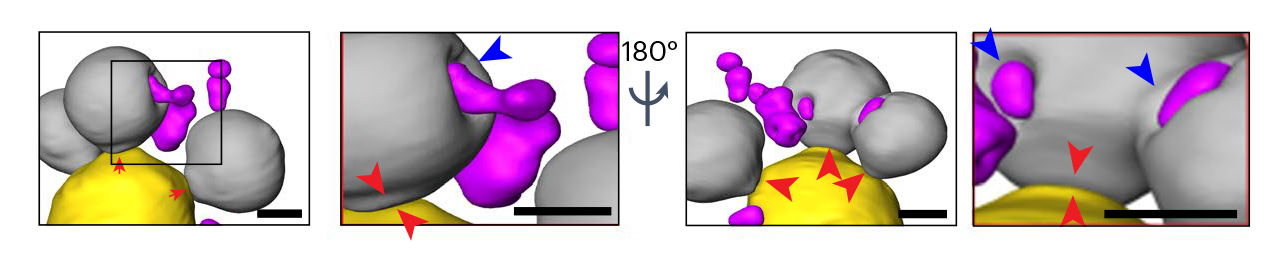
Cells can deal with misfolded proteins by refolding or eliminating them. While the cell decides whether to refold or degrade proteins, it sequesters them into membraneless inclusions—clusters of misfolded proteins, like tiny garbage dumps, that occur in both the cytoplasm and the nucleus. The researchers found that these inclusions migrate toward the boundary between the nucleus and a vacuole—a membrane-bound organelle full of enzymes for degrading proteins. Eventually, the nuclear and cytoplasmic misfolded protein inclusions line up to face each other, with the nuclear envelope separating them.
The team suspected that the reason for this was to send the inclusions to the vacuole for degradation. However, while it’s easy for cytoplasmic inclusions to enter the vacuole by autophagy—a process cells use to pull things from the cytoplasm into the vacuole—in the nucleus, inclusions are separated from the vacuole by the nuclear envelope.
From dump to degradation
To investigate the pathways of damaged proteins into the vacuole, the team blocked the proteasome—the other major protein-clearance mechanism—and monitored the remaining protein clearance activity. They also created images of cells containing these misfolded protein inclusions using cryogenic soft x-ray tomography at ALS Beamline 2.1 (the National Center for X-Ray Tomography) and super-resolution SIM.
The results showed that the cytoplasmic inclusions did push into the vacuole, as expected. But the route for the nuclear inclusions was surprising. The nuclear inclusions budded straight from the nucleus into the vacuole at the junction of the two membranes.
Using a series of genetic experiments, the team showed that a class of proteins used to create small vesicles for transporting molecules around cells (ESCRT II/III and Vps4 proteins) facilitated the budding-into-the-vacuole action. These proteins are known to cause membranes to bend and “bud,” or form new vesicles, in other processes, but have not been studied in the context of clearing the nucleus of damaged proteins. They may be attractive therapy targets for misfolded protein diseases.
One next step is to investigate whether this same pathway is used in mammalian cells to clear human disease-related proteins. Another is to define how communication between the nucleus and cytosol (the cytoplasmic liquid matrix) happens along the pathway, and yet another is to see how the pathway is affected by aging.
Contact: Emily Sontag
Researchers: E.M. Sontag (Marquette University and Stanford University); F. Morales-Polanco, T. Gestaut, and J. Frydman (Stanford University); J.H. Chen and G. McDermott (UCSF); P.T. Dolan (National Institute of Allergy and Infectious Diseases); and M.A. Le Gros and C. Larabell (UCSF and Berkeley Lab).
Funding: National Institutes of Health, Way Klingler Startup Funds (Marquette University), Pew Charitable Trusts, and the Gordon and Betty Moore Foundation. Operation of the ALS is supported by the US Department of Energy, Office of Science, Basic Energy Sciences program.
Publication: E.M. Sontag, F. Morales-Polanco, J.H. Chen, G. McDermott, P.T. Dolan, T. Gestaut, M.A. Le Gros, C. Larabell, and J. Frydman, “Nuclear and cytoplasmic spatial protein quality control is coordinated by nuclear–vacuolar junctions and perinuclear ESCRT,” Nat. Cell Biol. 25, 699 (2023), doi:10.1038/s41556-023-01128-6.
Adapted from the Stanford News article, “Study finds new pathway for clearing misfolded proteins.”
ALS SCIENCE HIGHLIGHT #488