SCIENTIFIC ACHIVEMENT
Researchers found that a small-molecule protein inhibitor—screened from billions of compounds and analyzed using structural insights from protein crystallography performed at the Advanced Light Source (ALS)—reversibly suppresses male fertility in mice.
SIGNIFICANCE AND IMPACT
The work addresses the pressing need for more contraceptive options that enable all individuals to control their own fertility.
Eight billion and counting
The United Nations designated November 15, 2022, as the Day of Eight Billion, the day the population of our planet passed eight billion people. This number is projected to reach ten billion by 2050. A sobering statistic is that nearly half of all pregnancies worldwide are unintended, which results in emotional, physical, and financial burdens on individuals as well as health care systems.
Contraception enables individuals to choose when and whether to conceive. But more options are needed—for nonhormonal contraceptives (which target specific proteins related to reproduction) and for non-barrier, reversible methods for men.
Here, researchers identified a molecule that blocks an enzyme key to male fertility. Using protein crystallography, they gained valuable structure–activity insight and, in tests on mice, showed that an optimized version of the compound is both effective and reversible.
Shining light on a “dark kinase”
The protein target in this work is an enzyme called serine/threonine kinase 33 (STK33). Kinases activate cellular processes and are therefore prime targets for drug therapies. However, there are over 500 human kinases, and many—sometimes called “dark kinases”—are under-researched and poorly understood. STK33 fell into this category, until a study of men with STK33 mutations and experiments in which the STK33 gene was nullified in mice established that deactivating STK33 in males suppresses fertility.
To identify small-molecule drug candidates for blocking STK33, the researchers used a high-throughput screening process whereby each candidate molecule was tagged with a unique DNA sequence. This enabled the screening of billions of molecules together with STK33 in a single test tube. Compounds that ended up tightly bound to the protein (“hits”) could then be identified by sequencing the DNA tags.
Structure-guided design
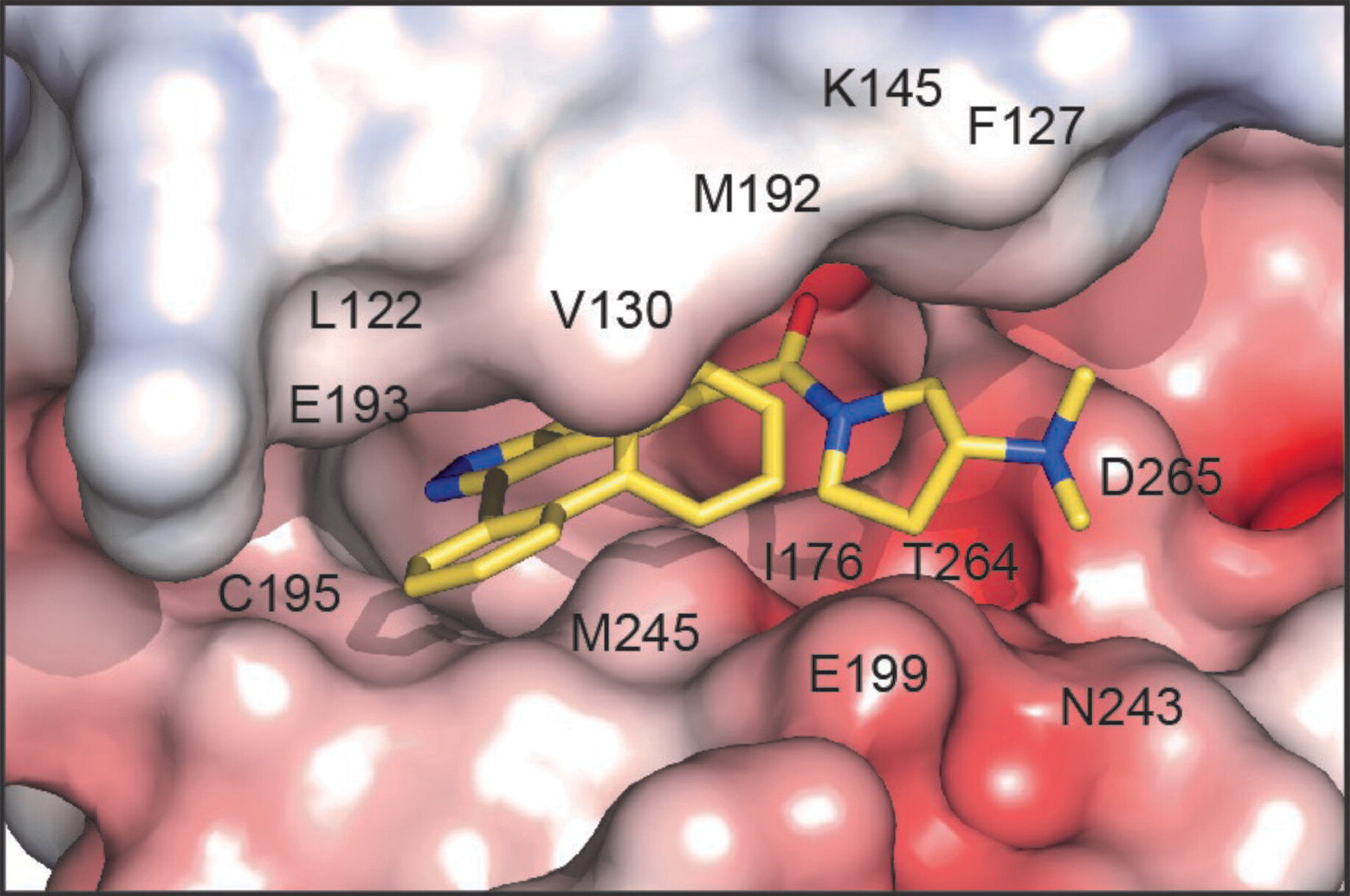
To understand the molecular basis for high-affinity STK33 inhibitors, the researchers used protein crystallography at ALS Beamline 5.0.2 to determine the structure of STK33, co-crystallized with a hit from the screening process (CDD-2211) that proved amenable to crystallization.
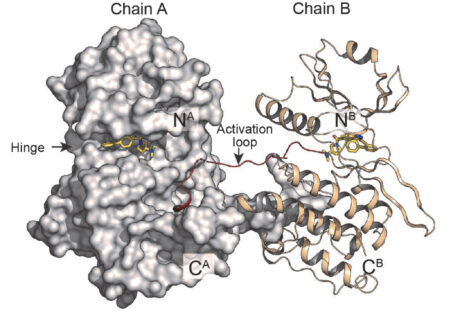
Because the structure of STK33 had never been previously determined, the researchers were surprised to discover that it formed a dimer. The data also settled the question of how molecules are oriented in the binding pocket, after predictive models had given two different possibilities. Specific interactions between the molecule and the protein’s amino acids showed why CD-2211 has such high affinity for STK33. This information will prove very useful in tailoring compounds for greater STK33 specificity.
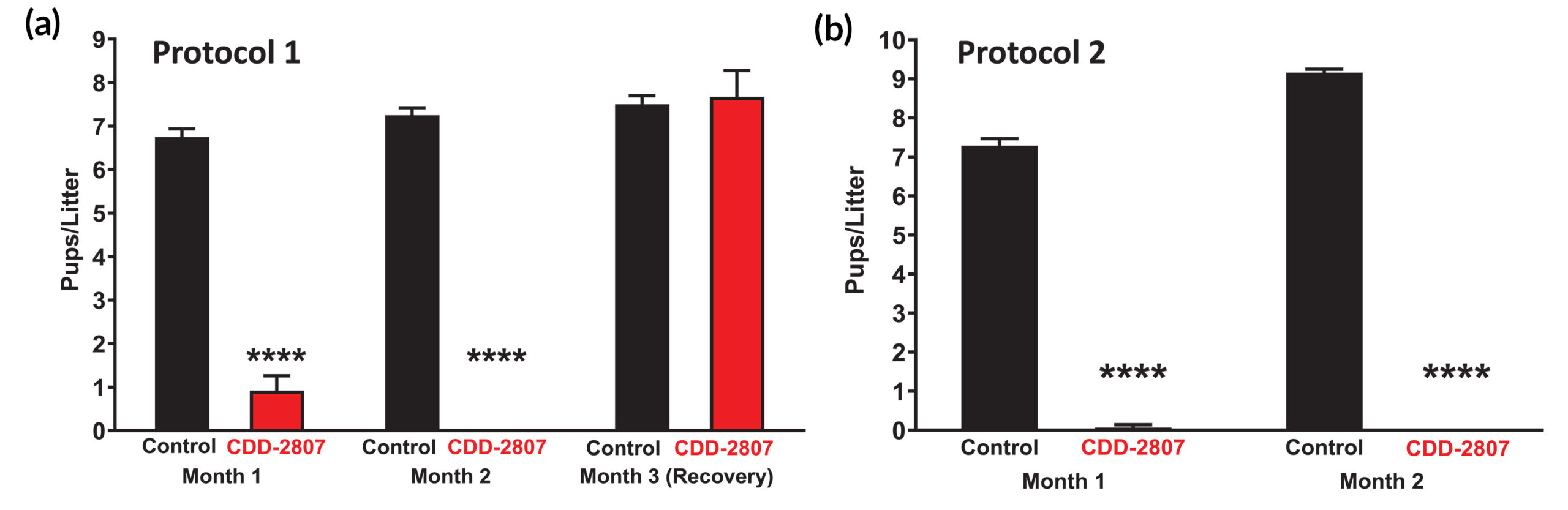
Encouraging tests in mice
Finally, to determine the consequences of STK33 inhibition in vivo, the researchers evaluated reproductive outcomes in male mice dosed with CDD-2807, another strong hit from the screening process that’s structurally similar to CDD-2211 but exhibits better metabolic stability. Two dosing protocols were followed: injecting a lower dose twice a day and a higher dose once a day. In both cases, the results showed that CDD-2807 exhibited no toxicity, efficiently crossed the blood-testis barrier, did not accumulate in brain, and induced a reversible contraceptive effect. The researchers are currently working on analogs of CDD-2807 that would be orally bioavailable.
Contact: Martin Matzuk
Researchers: A.F. Ku, K.L. Sharma, H.M. Ta, C.M. Sutton, K.M. Bohren, Y. Wang, S. Chamakuri, R. Chen, J.M. Hakenjos, R. Jimmidi, K. Kent, F. Li, J.-Y. Li, L. Ma, C. Madasu, M. Palaniappan, S.S. Palmer, X. Qin, Z. Tan, Y.M. Vasquez, J. Wang, Z. Yu, Q. Ye, D.W. Young, M. Teng, C.W. Kim, and M.M. Matzuk (Baylor College of Medicine); M.B. Robers and J. Wilkinson (Promega Corporation); and B. Sankaran (Berkeley Lab).
Funding: Bill and Melinda Gates Foundation, A.I. and Manet Schepps Discovery Foundation, National Institutes of Health, Cancer Prevention Research Institute of Texas. Operation of the ALS is supported by the US Department of Energy, Office of Science, Basic Energy Sciences program.
Publication: A.F. Ku, K.L. Sharma, H.M. Ta, C.M. Sutton, K.M. Bohren, Y. Wang, S. Chamakuri, R. Chen, J.M. Hakenjos, R. Jimmidi, K. Kent, F. Li, J.-Y. Li, L. Ma, C. Madasu, M. Palaniappan, S.S. Palmer, X. Qin, M.B. Robers, B. Sankaran, Z. Tan, Y.M. Vasquez, J. Wang, J. Wilkinson, Z. Yu, Q. Ye, D.W. Young, M. Teng, C.W. Kim, and M.M. Matzuk, “Reversible male contraception by targeted inhibition of serine/threonine kinase 33,” Science 384, 885 (2024), doi:10.1126/science.adl2688.
ALS SCIENCE HIGHLIGHT #509