SCIENTIFIC ACHIEVEMENT
A promising anticancer drug, AMG 510, was developed by Amgen with the help of novel structural insights gained from protein structures solved at the Advanced Light Source (ALS).
SIGNIFICANCE AND IMPACT
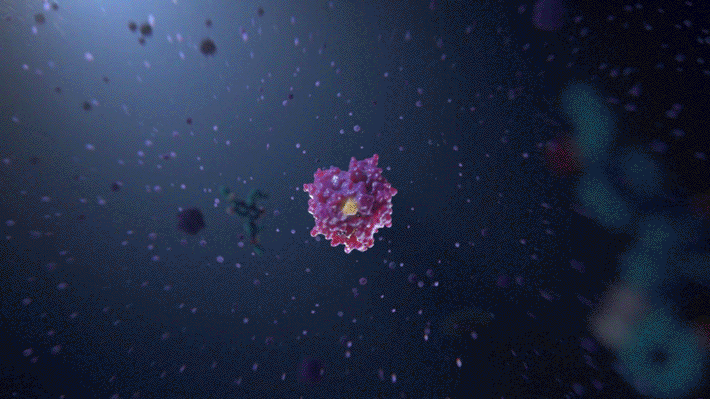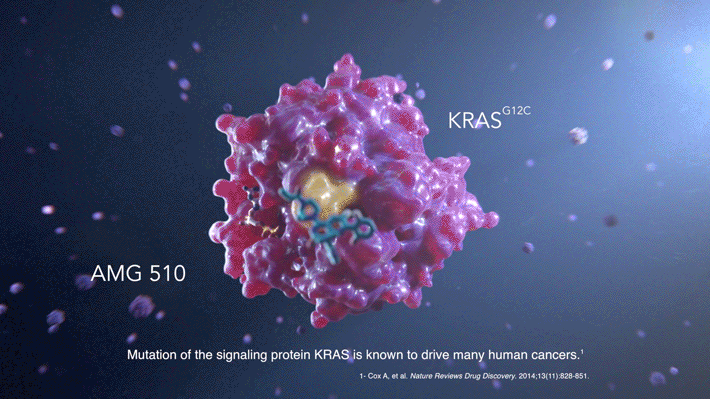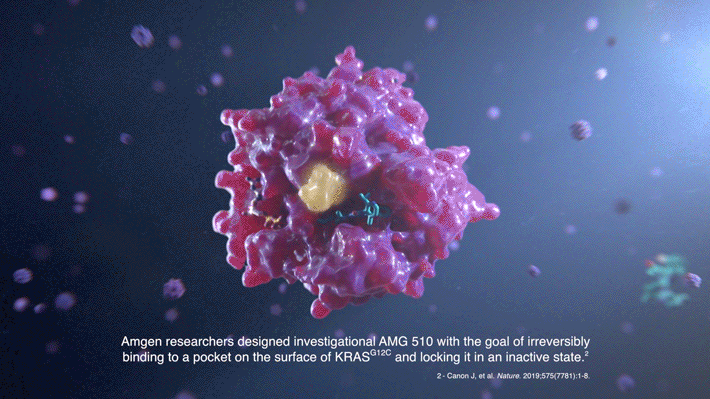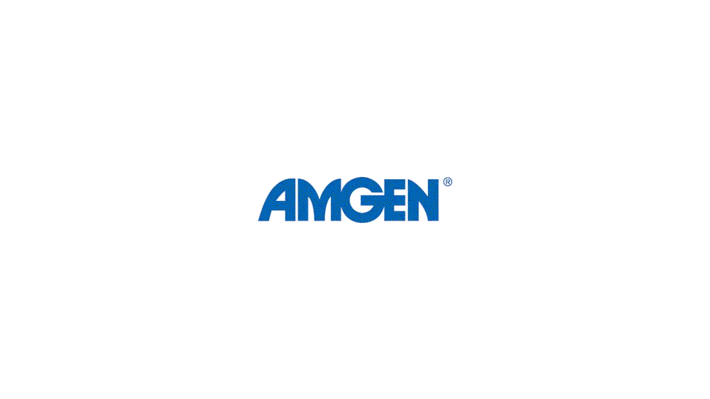
AMG 510, which is currently in phase II clinical trials for efficacy, targets tumors caused by mutations in the KRAS protein, one of the most common causes of cancer.
An “undruggable” cancer trigger?
Mutations in a signaling protein, KRAS, are known to drive many human cancers. One specific KRAS mutation, KRAS(G12C), accounts for approximately 13% of non-small cell lung cancers, 3% to 5% of colorectal cancers, and 1% to 2% of numerous other solid tumors. Approximately 30,000 patients are diagnosed each year in the United States with KRAS(G12C)-driven cancers.
Despite their cancer-triggering significance, KRAS proteins have for decades resisted attempts to target their activity, leading many to regard these proteins as “undruggable.” Recently, however, a team led by researchers from Amgen identified a small molecule capable of inhibiting the activity of KRAS(G12C) and driving anti-tumor immunity. Protein crystallography studies at the ALS provided crucial information about the structural interactions between the potential drug molecule and KRAS(G12C).
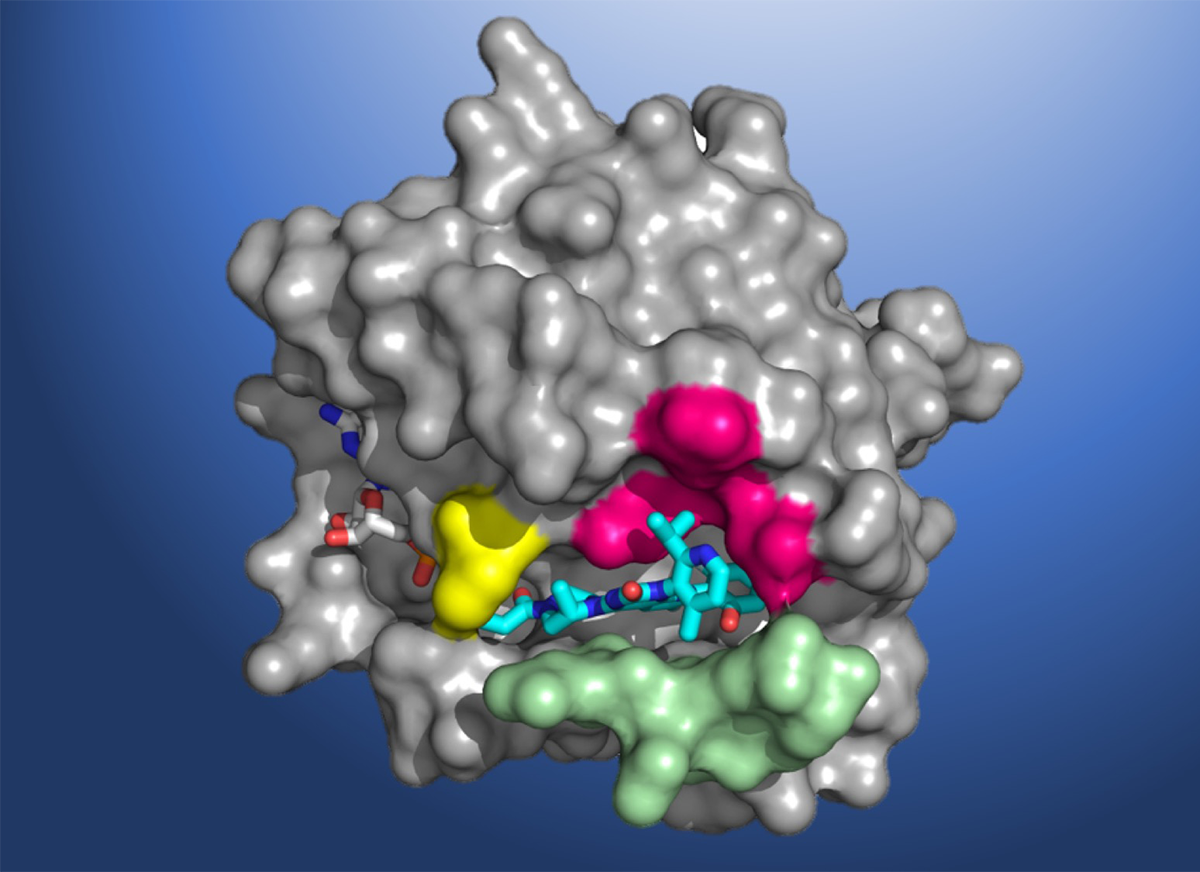
Discovery of a cryptic groove
Designing KRAS-specific therapeutic drugs is difficult because of the shape of the protein—it has an exceptionally smooth surface with few obvious regions where a drug molecule could bind. At ALS Beamline 5.0.1, part of the Berkeley Center for Structural Biology, Amgen researchers used x-ray crystallography to obtain high-resolution structural maps of KRAS(G12C) co-crystallized with various small molecules (ligands).
The key breakthrough was the discovery of a “cryptic” surface groove—a normally undetectable indentation that only becomes evident upon ligand binding. In this case, alternate positioning of an amino acid, histidine 95 (His95), was found to reveal the surface groove. Optimizing ligands that favorably occupy this groove was key to achieving drug-like levels of inhibitory activity toward KRAS(G12C). The beamline data allowed scientists to investigate atomic-level molecular interactions between KRAS(G12C) and potential inhibiting compounds that bind in this pocket.
While earlier seminal work by Shokat and colleagues led to the development of tool inhibitors—compounds demonstrating that G12C could be targeted by small-molecule inhibition in preclinical studies—the discovery of the His95 groove facilitated the development of G12C inhibitors with substantially enhanced potency and selectivity, making them suitable for clinical development.
Development and testing of AMG 510
Through extensive compound screening and structure-based design, a compound called AMG 510 emerged as the top candidate for binding the His95 groove, combining both improved potency and selectivity. It is designed to irreversibly bind to the KRAS(G12C) protein and permanently lock it in an inactive state.
In preclinical experiments in mice, AMG 510 was found to durably inhibit tumor-cell growth. Follow-up studies suggested that, not only could AMG 510 work synergistically with immunotherapy approaches, it could also improve the anti-tumor efficacy of chemotherapy.
The enhanced potency and efficacy of AMG 510 prompted its selection as the first KRAS(G12C) inhibitor to enter phase I clinical trials (for safety), with promising results. The compound has now begun phase II studies (for efficacy), potentially generating data that can be eventually used in the regulatory approval process. For now, in early clinical trials, AMG 510 has demonstrated anti-tumor activity in the first dosing cohorts and represents a potentially transformative therapy for patients for whom effective treatments are currently lacking.
Contact: Christopher Mohr
Researchers: J. Canon, K. Rex, A.Y. Saiki, C. Mohr, K. Cooke, D. Bagal, K. Gaida, T. Holt, C.G. Knutson, N. Koppada, B.A. Lanman, J. Werner, A.S. Rapaport, T. San Miguel, R. Ortiz, T. Osgood, J.-R. Sun, X. Zhu, J.D. McCarter, L.P. Volak, B.E. Houk, W. Ouyang, H. Henary, T. Arvedson, V.J. Cee, and J.R. Lipford (Amgen Inc.); M.G. Fakih (City of Hope Comprehensive Cancer Center); B.H. O’Neil (Indiana University); T.J. Price (The Queen Elizabeth Hospital and University of Adelaide, Australia); G.S. Falchook (Sarah Cannon Research Institute); J. Desai (Peter MacCallum Cancer Center, Australia); J. Kuo (Scientia Clinical Research, Australia); R. Govindan (Washington University in St. Louis); and D.S. Hong (The University of Texas MD Anderson Cancer Center).
Funding: Amgen Inc., National Institutes of Health, and Howard Hughes Medical Institute. Operation of the ALS is supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences Program.
Publication: J. Canon, K. Rex, A.Y. Saiki, C. Mohr, K. Cooke, D. Bagal, K. Gaida, T. Holt, C.G. Knutson, N. Koppada, B.A. Lanman, J. Werner, A.S. Rapaport, T. San Miguel, R. Ortiz, T. Osgood, J.-R. Sun, X. Zhu, J.D. McCarter, L.P. Volak, B.E. Houk, M.G. Fakih, B.H. O’Neil, T.J. Price, G.S. Falchook, J. Desai, J. Kuo, R. Govindan, D.S. Hong, W. Ouyang, H. Henary, T. Arvedson, V.J. Cee, and J.R. Lipford, “The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity,” Nature 575, 217 (2019), doi: 10.1038/s41586-019-1694-1.
Adapted from the Berkeley Lab snapshot, “Unique Cancer Drug Discovered With Help From Advanced Light Source Begins Historic Clinical Trial.”
Read more about the development of AMG 510 and its early clinical results in the Amgen press release, “The Discovery Of Amgen’s Novel Investigational KRAS(G12C) Inhibitor AMG 510 Published In Nature.”
ALS SCIENCE HIGHLIGHT #412