Most people have heard of adrenaline, the chemical that causes the “fight or flight” reaction in humans. Most people have also heard of the chemical substances cocaine and methamphetamine, which also elicit a particular (perhaps desired) human response. What most people do not know is that the same receptors in the human brain recognize the natural, or endogenous, chemicals produced by the brain (such as dopamine, serotonin, and noradrenaline) and the unnatural ones that cross the blood-brain barrier, such as cocaine and antidepressants. That is in fact why those non-endogenous substances “work” so well—they mimic just well enough our natural chemistry that our brains readily recognize and respond to them.
The amine transporters are one of the brain’s gatekeepers for these chemicals; they reside in the terminal endings of brain cells and their job is to recognize dopamine, serotonin, and several other endogenous molecules. Depending on the chemical detected, they set in motion a cascade of brain activity.
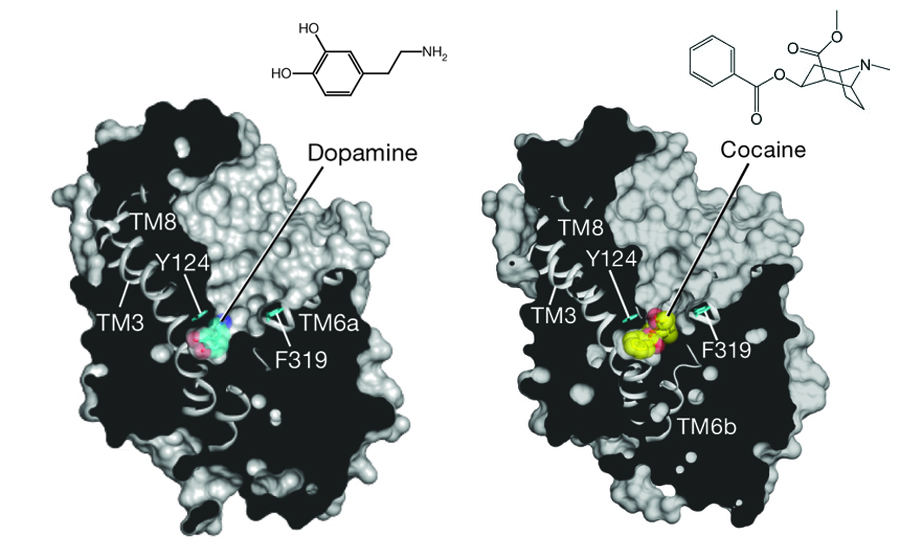
Dopamine, in particular, is associated with “feeling good.” Its release in the brain is caused by reward-related behaviors. Cocaine works by blocking the re-uptake of dopamine (and other neurotransmitters) so that dopamine remains in the brain, maintaining that “reward” feeling. After repeated cocaine consumption, however, the brain responds by producing less dopamine; this means that gradually more cocaine is needed to achieve the same feeling, leading to addiction. Loss of dopamine secretion is also associated with diseases such as Parkinson’s and other neurological conditions. Clearly, maintaining at all times the proper levels of neurotransmitters such as dopamine is critical for human health. So why do our brains respond so well to unnatural drugs like cocaine? The molecular structures of dopamine and cocaine, after all, are not very similar.
Scientists working at ALS Beamlines 8.2.1 and 5.0.2 recently solved the crystallographic structures of the dopamine transporter bound to several of these non-endogenous chemicals in order to try to find the answer to why the human brain responds to those chemicals. What they found was very interesting: antidepressants and cocaine don’t necessarily fit into the binding pocket designed for dopamine, but instead cause the binding pocket to change shape. One of the transmembrane helices in the protein, TM6, which spans the length of the protein from inner to outer cell membrane layer, is interrupted by the dopamine binding site and held together with a short linker region. This linker shifts to accommodate dopamine binding, and at the same time, one of the protein sidechains rotates in to contract the size of the binding pocket. It is this “plasticity” in the binding site that allows bulkier chemicals to enter the binding site as well. For instance, nortriptyline (an antidepressant) causes that same sidechain to rotate out, making the binding pocket larger to accommodate the chemical. The larger chemicals in effect “wedge” the binding pocket open, which explains how they act as inhibitors for dopamine re-uptake.
Why the transporter shifts and contorts to handle non-endogenous chemicals is not yet answered, but the “how” of the question is partially answered. In any case, knowledge of how these chemicals bind to amine transporters will help in the design of drugs to treat many neurological diseases, and may also lead to a better understanding of how addiction to abused drugs such as cocaine can be managed.
Contact: Eric Gouaux
Research conducted by: K.H. Wang and A. Penmatsa (Oregon Health & Science University) and E. Gouaux (Oregon Health & Science University, Howard Hughes Medical Institute).
Research funding: U.S. Department of Energy (DOE), Office of Basic Energy Sciences (BES) and Howard Hughes Medical Institute. Operation of the ALS is supported by DOE BES.
Publication about this research: K.H. Wang, A. Penmatsa, and E. Gouaux, “Neurotransmitter and psychostimulant recognition by the dopamine transporter,” Nature 521 (2015).
ALS SCIENCE HIGHLIGHT #321