Using a computer-based approach, researchers designed porous protein crystals that were revealed to be stable, tunable, and atomically accurate using x-ray scattering and diffraction at the ALS. The work provides a powerful new platform for biological materials engineering and opens up wide applications in biotechnology and medicine. Read more »![]()
![]()
Chemical acylation of an acquired serine suppresses oncogenic signaling of K-Ras(G12S)
Small-molecule targeting of particular KRAS mutations offer promise for cancer therapy. The cover depicts a small-molecule ligand (red) inhibiting the oncogenic mutant protein K-Ras(G12S) (cyan) by forming a covalent ester adduct at the mutant serine. Read more »
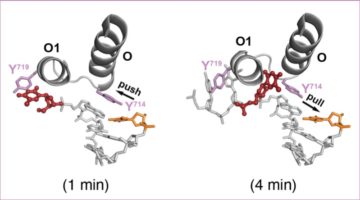
Molecular Switch Triggers Changes in Plant Structure
Using x-ray crystallography, biochemistry, and plant genetics, researchers identified a molecular switch that triggers modifications to plant structure in response to environmental conditions. A greater understanding of this adaptive process will help scientists optimize plants for efficient nutrient uptake and resistance to parasitic species. Read more »![]()
![]()
DNA Synthesis: Flip It and Reverse It
What if the current model for DNA synthesis were flipped on its head? Using time-resolved x-ray crystallography, researchers gained new insights into this essential biological process, revealing that two steps in the synthesis pathway are, in reality, reversed. Read more »
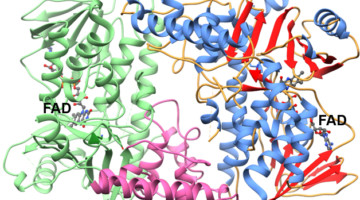
Functional and structural characterization of AntR, an Sb(III) responsive transcriptional repressor
Antimony is considered a priority environmental pollutant by the EPA. The ant operon of the antimony-mining bacterium, C. testosterone, confers resistance to Sb(III). The operon is regulated by the product of the first gene in the operon, antR. This is the first report of the structure and binding properties of antR, with high selectivity for environmental antimony. Read more »
Jennifer Doudna and the Nobel Prize: The Advanced Light Source Perspective
The 2020 Nobel Prize in Chemistry was awarded to Jennifer Doudna and Emmanuelle Charpentier for the development of a world-changing gene-editing technology. At the ALS, Doudna’s work on CRISPR-Cas9 was enabled by many visionary people with innovative ideas, implemented in support of a world-class structural biology program. Read more »![]()
Providing New Technologies for Vaccine Development
Antigens can sometimes be attached to a protein scaffold to mimic the shape of a virus and elicit a stronger immune response. Scientists developed a method to design such proteins, and ALS data helped to visualize the atomic structure and determine the dynamics of the designed scaffolds. Read more »
Crystal structure of the catalytic subunit of bovine pyruvate dehydrogenase phosphatase
The crystal structure of the catalytic subunit of bovine pyruvate dehydrogenase phosphatase provides new insights into the mechanism of the regulation of the activity of the pyruvate dehydrogenase complex. Read more »
Genetic Blueprint for the Bioproduction of an Antidepressant Drug Candidate
A set of genes from a marine bacterium has been found to encode the biosynthesis of a promising antidepressant drug candidate. This work, which used the ALS to solve the structure of a key enzyme, could enable industrial-scale bioproduction of the drug in ways that are more efficient and sustainable than chemical synthesis. Read more »
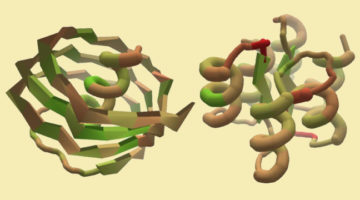
A Citizen-Science Computer Game for Protein Design
Using the computer game, “Foldit,” nonexpert citizen scientists designed new proteins whose structures, verified at the ALS, were equivalent in quality to and more structurally diverse than computer-generated designs. The work shows the potential of using crowd-based creativity in the design of new proteins for fighting illness and disease. Read more »![]()
![]()