With an aging population in America, it is more important than ever to discover ways to treat or prevent diseases affecting the brain and its ability to make new connections and recall memories. Some of the most important players in the brain’s normal function are the glutamate receptors, which are involved in nervous-system development and function. These molecules transmit signals between nerve cells and are critical to learning and memory. Mutations in this family of ion-channel molecules are associated with neurological and neuropsychiatric conditions such as Parkinson’s disease and autism. Glutamate receptors are already the target for therapeutics aimed at treating epilepsy, depression, Alzheimer’s disease, and more.
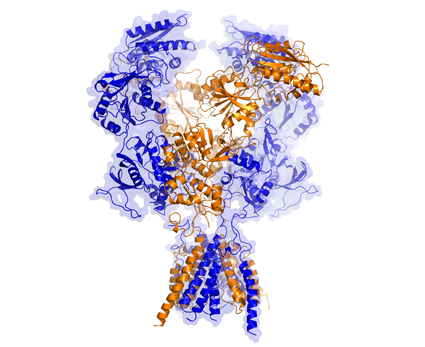
305gouaux Multiple structures of two glutamate receptors, AMPA and NMDA, were solved by x-ray crystallography in the Berkeley Center for Structural Biology (BCSB) at the ALS by Eric Gouaux and his team from the Oregon Health & Science University’s Vollum Institute. Each receptor is named for the synthetic molecule that it binds more tightly than glutamate (the primary neurotransmitter in the brain): AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; and NMDA, N-methyl-D-aspartate. The receptors used in the studies—from the Norwegian rat and the frog Xenopus laevis, respectively—are essentially identical or very similar in function to their human analogues and are thus highly relevant to understanding their function in humans.
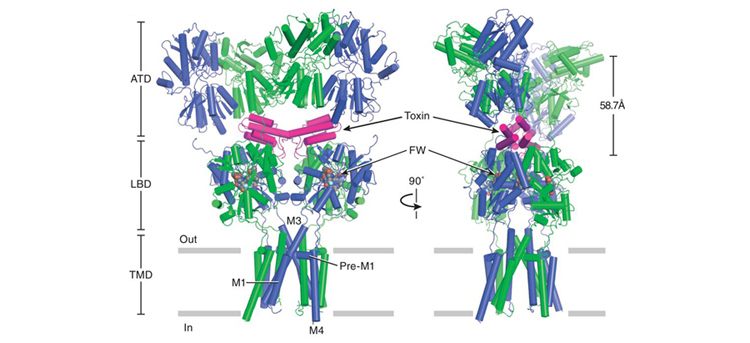
AMPA binds to small molecules, or ligands, that activate its ion-channel gating activity. In a study published in Science last August, Gouaux and his colleagues describe several AMPA structures, with and without mutations, bound to a cone-snail toxin in addition to other small molecules that affect receptor structure and function. The cone-snail toxin (con-ikot-ikot) is a disulfide-bond-rich polypeptide, previously shown to induce paralysis in fish and to potently and selectively affect the sensitization of AMPA receptors to the presence of glutamate. The structures of these complexes provide insight into the activation mechanism of this receptor, as well as its many interactions with an AMPA-specific toxin.
NMDA is both ligand-gated and voltage-dependent, meaning that the extracellular portion of this receptor triggers ion-channel pore activation when glutamate and another ligand bind to the receptor, allowing the flow of calcium ions. Gouaux and his fellow researchers reported two structures in a July 2014 Nature article that give rise to a deeper understanding of this glutamate receptor. “The NMDA receptor is one of the most important receptors in our brain, and yet we are still discovering how it works,” said Gouaux, senior scientist and Howard Hughes Medical Investigator. “With these structures, we can see incredible detail and lay the groundwork for the development of future therapeutics.”

Gouaux’s team solved the structures using BCSB Beamlines 8.2.1 and 5.0.2 and refined them using Phenix x-ray crystallography software developed in Berkeley Lab’s Physical Biosciences Division. AMPA and NMDA have similar architectures, possessing an amino-terminal domain and a ligand-binding domain, both located outside of the cell, and a transmembrane domain that spans the membrane and defines the ion-channel pore. NMDA has an additional carboxy-terminal domain inside the cell. Researchers made amino-acid-sequence modifications to both receptors to improve stability for crystallization while retaining the ability to bind small molecules and maintain biological activity when exposed to ligands.
These structures provide a molecular blueprint for use in developing new therapeutics, as well as a structural framework for understanding how these molecules are modulated and function as ion channels that pass neural signals through cell membranes. The information can lead to greater understanding, not only of the AMPA and NMDA receptors, but of the kainate receptor as well, which is in the same class of glutamate receptors. The knowledge gained from the detailed molecular structures of both receptors will be invaluable as researchers develop drugs designed to treat or cure numerous debilitating neurological diseases and conditions.
Contact: Eric Gouaux
Research conducted by: L. Chen, K.L. Duerr, C.H. Lee, W. Lü, J. Carlisle Michel, A. Goehring, J. Du, X. Song, and E. Gouaux (Oregon Health & Science University).
Research funding: American Heart Association, European Molecular Biology Organization, Oregon Brain Institute, National Institutes of Health, Vollum Institute, and Howard Hughes Medical Institute. Operation of the ALS is supported by the U.S. Department of Energy (DOE), Office of Basic Energy Sciences (BES).
Publications about this research: L. Chen, K.L. Duerr, and E. Gouaux, “X-ray structures of AMPA receptor–cone snail toxin complexes illuminate activation mechanism,” Science 345, 1021 (2014), doi:10.1126/science.1258409; and C.-H. Lee, W. Lü, J. Carlisle Michel, A. Goehring, J. Du, X. Song, and E. Gouaux, “NMDA receptor structures reveal subunit arrangement and pore architecture,” Nature 511, 191 (2014); doi:10.1038/nature13548.
ALS SCIENCE HIGHLIGHTS #305