Developing electrical-energy storage systems with high energy density and low cost is extremely important for powering society in the future. As one of the dominant energy-storage devices, lithium-ion batteries have been widely applied in portable electronic devices and electric vehicles. However, the capacities of conventional electrode materials for lithium-ion batteries are approaching their theoretical limits; therefore, it is essential to develop new high-energy electrode materials.
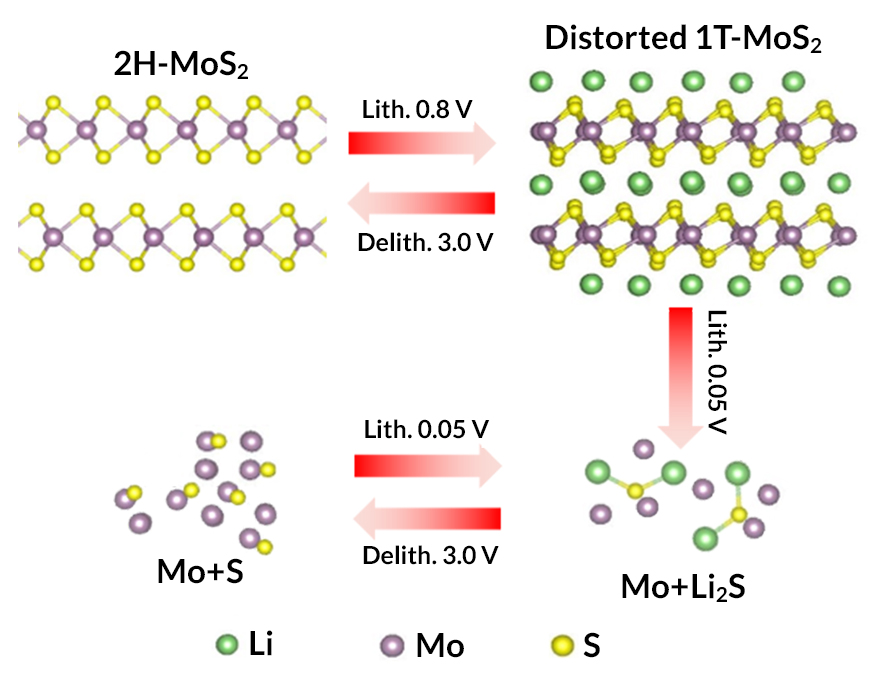
Two-dimensional transition metal dichalcogenides (TMDs) have attracted great scientific interest in electrochemical energy storage recently, owing to their unique layered structures and tunable electronic and chemical properties. As a typical TMD, layered molybdenum disulfide (MoS2) is regarded as a promising electrode for high-energy lithium-ion batteries. However, despite decades of effort and intensive recent interest, the lithiation/delithiation reaction mechanism of MoS2 is still controversial, mainly due to a lack of suitable detection methods.
At ALS Beamlines 5.3.1 and 10.3.2, researchers studied the reaction mechanism of MoS2 electrodes in lithium-ion battery cells under real operating conditions using x-ray absorption spectroscopy (XAS). The results reveal that the electrode experiences lithium intercalation and conversion reactions sequentially during the first discharge. However, the conversion reaction is not reversible, and the formed Li2S is oxidized to sulfur in the subsequent charge process, clarifying a previous debate about the reversibility of the MoS2 conversion reaction. The work enriches our fundamental understanding of the electrochemical reaction mechanism of the MoS2 electrode, a crucial step toward the rational design of TMD electrodes with superior cycling performance.
L. Zhang, D. Sun, J. Kang, J. Feng, H.A. Bechtel, L.-W. Wang, E.J. Cairns, and J.-H. Guo, “Electrochemical Reaction Mechanism of the MoS2 Electrode in a Lithium-Ion Cell Revealed by in Situ and Operando X-ray Absorption Spectroscopy,” Nano Lett. 18, 1466 (2018), doi:10.1021/acs.nanolett.7b05246.