For over ten years, ALS Beamline 8.0.1 has been a prime destination for scientists interested in studying that most basic of substances: water. Despite the fundamental importance of water to biology and chemistry, researchers have found it extremely challenging to study. Many of water’s more unusual properties (high heat capacity, solvent strength, surface tension, etc.) involve its propensity to form hydrogen bonds, which, despite being relatively weak, are absolutely essential to life on Earth.

A team of scientists from the Karlsruhe Institute of Technology (KIT); the University of Heidelberg; the University of Würzburg; the University of Nevada, Las Vegas (UNLV); and the ALS is specifically interested in how water molecules interact with each other and with other substances via hydrogen bonds. “What we are trying to understand is the electronic structure of liquids and solutions, and in particular, the interaction between different constituents,” says Lothar Weinhardt, head of the spectroscopy division of the Institute for Photon Science and Synchrotron Radiation (IPS) at KIT. “Here, we were investigating such interactions in aqueous ammonia and salt solutions.”

Weinhardt and his colleagues have recently published a series of papers that open up the possibility of probing hydrogen bonds in aqueous solutions by combining x-ray emission spectroscopy (XES) and resonant inelastic soft x-ray scattering (RIXS), using the specialized Solid and Liquid Spectroscopic Analysis (SALSA) endstation at Beamline 8.0.1.
“These are quite challenging experiments,” says Weinhardt. “The techniques typically need an ultrahigh vacuum environment, so we need an experimental set up where we can separate the vacuum from our liquid. Also, x-ray emission is a technique that has a very low cross section in the soft x-ray range, so we need something that gives us a lot of photons and excitations—a very bright source.”
According to Beamline Scientist Wanli Yang, “RIXS has always been known as a photon-hungry technique,” and the need for high flux is even more crucial for in situ experiments, due to their reduced signal-to-noise ratio. “Beamline 8.0.1 is a high-flux undulator beamline at a synchrotron optimized for the soft x-ray range,” he says. “Therefore, the beamline is naturally one of the most suitable places to run soft x-ray RIXS in situ experiments.”
Compared to older setups at Beamline 8.0.1, SALSA improves the detection efficiency by one to two orders of magnitude, without sacrificing energy resolution. “With such a high efficiency,” says Weinhardt, “you can do things that you were not able to do before. We basically combine x-ray emission spectroscopy and x-ray absorption spectroscopy at the same beamline at the same time.”
In conventional RIXS studies, only a few resonant emission spectra are collected at selected excitation energies. “What we do here,” says Weinhardt, “is measure a full map—a dense grid of these spectra.” The resulting 2D maps make it much easier to distinguish between the redistribution of intensity between several components on one hand, and energy shifts in a single component on the other.
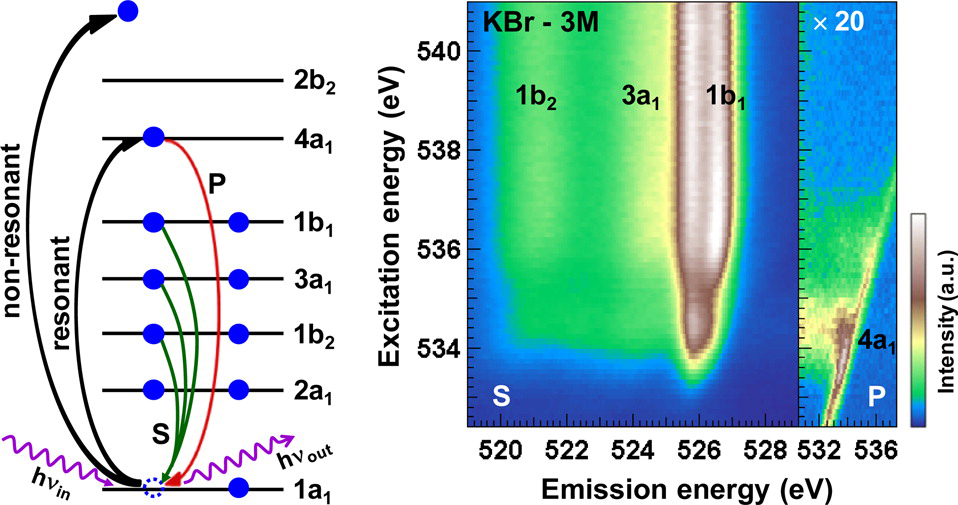
As motivation for the experiments involving salts, Weinhardt cites the example of the Hofmeister series. Over a century ago, a chemist named Franz Hofmeister discovered that various salts can be ranked according to their ability to cause proteins in aqueous solutions to “salt in” (dissolve) or “salt out” (precipitate). This useful yet seemingly random ordering is very important in biology, but the explanation for it is still a mystery whose solution seems tantalizingly within reach using techniques that probe the electronic structure of water molecules.
“Different things could be happening,” explains Weinhardt. “Apart from the formation of hydration shells, the salt might either change the properties of the water over long ranges or directly act on the protein in the short range.”
Overall, Weinhardt’s team will continue working at Beamline 8.0.1, increasing the complexity of the systems under investigation. Having started with pure water, they have now progressed to water with ions (dissolved salts) and small molecules (ammonia). “Now we are moving on to larger molecules,” says Weinhardt. “Step-by-step, you work your way up to something like peptides or proteins, systems that are more directly relevant to biological questions. So it’s very important to really understand all the details of these spectra of simpler systems.”
Y.L. Jeyachandran, F. Meyer, A. Benkert, M. Bär, M. Blum, W. Yang, F. Reinert, C. Heske, L. Weinhardt, and M. Zharnikov, “Investigation of the Ionic Hydration in Aqueous Salt Solutions by Soft X-ray Emission Spectroscopy,” J. Phys. Chem. B 120, 6787 (2016). doi:10.1021/acs.jpcb.6b03952
L. Weinhardt, E. Ertan, M. Iannuzzi, M. Weigand, O. Fuchs, M. Bär, M. Blum, J.D. Denlinger, W. Yang, E. Umbach, M. Odelius, and C. Heske, “Probing hydrogen bonding orbitals: resonant inelastic soft X-ray scattering of aqueous NH3,” Phys. Chem. Chem. Phys. 17, 27145 (2015). doi:10.1039/c5cp04898b
Y.L. Jeyachandran, F. Meyer, S. Nagarajan, A. Benkert, M. Bär, M. Blum, W. Yang, F. Reinert, C. Heske, L. Weinhardt, and M. Zharnikov, “Ion-Solvation-Induced Molecular Reorganization in Liquid Water Probed by Resonant Inelastic Soft X-ray Scattering,” J. Phys. Chem. Lett. 5, 4143 (2014). doi:/10.11021/jz502186a