SCIENTIFIC ACHIEVEMENT
Researchers electrochemically reduced CO2 to CO with nearly perfect selectivity over other products by adding an organic compound to the surface of a silver electrode.
SIGNIFICANCE AND IMPACT
With theoretical analyses and Advanced Light Source (ALS) data, the work revealed the key role of the microenvironment in promoting the conversion of CO2, a greenhouse gas, into useful products.
Bending the carbon curve
Carbon dioxide (CO2) makes up 80% of the greenhouse gases released into the atmosphere by the United States, mainly through the burning of fossil fuels. After a slight dip last year during the pandemic, the trend for CO2 emissions is again on the rise. One thing we can do to help bend this curve downward again is to capture CO2 and convert it into high-value feedstock chemicals such as ethylene and ethanol, which are chemical building blocks for other useful products.
Copper is best, silver is simpler
After years of searching for a material that improves on copper’s performance for the conversion of CO2 into high-value chemicals, scientists found that copper remains the best overall. However, it still isn’t efficient enough for commercial applications. So, the focus shifted away from finding a better electrode material and toward re-engineering the microenvironment at the electrode’s surface.
Silver, it turns out, is very good at converting CO2 to CO, which is the first step in the process for producing higher hydrocarbons. However, silver isn’t as versatile as copper—it only produces CO and hydrogen gas (H2). While this simplicity is a negative in terms of practical applications, it’s a positive in terms of disentangling how specific changes to the microenvironment affect performance.
Astounding improvements in selectivity
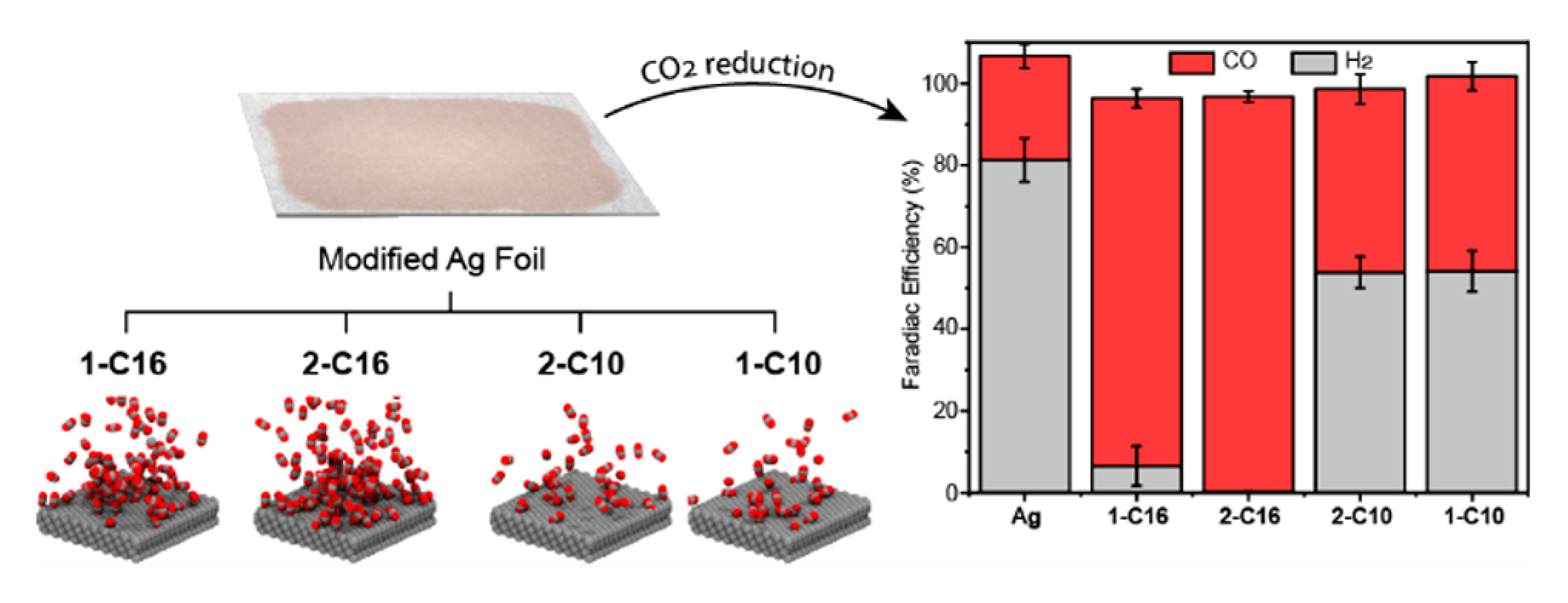
In this work, researchers drew from previous experiments in which they found that certain organic additives can tune copper’s ability to reduce CO2. To clarify the mechanism behind this observation, they repeated the experiment with a less complicated system, silver. Four organic compounds were tested: a series of alkylammonium salts, characterized by one or two long carbon chains with either 10 or 16 carbons (1-C10, 2-C10, 1-C16, and 2-C16).
The researchers discovered that 2-C16 increased the production of CO from CO2 by a factor of 9 and decreased the production of H2 by a factor of 440—a dramatic shift in selectivity for CO. To better understand the reasons for this astounding improvement, the researchers turned to theoretical simulations combined with x-ray scattering experiments at the ALS.
Understanding the microenvironment
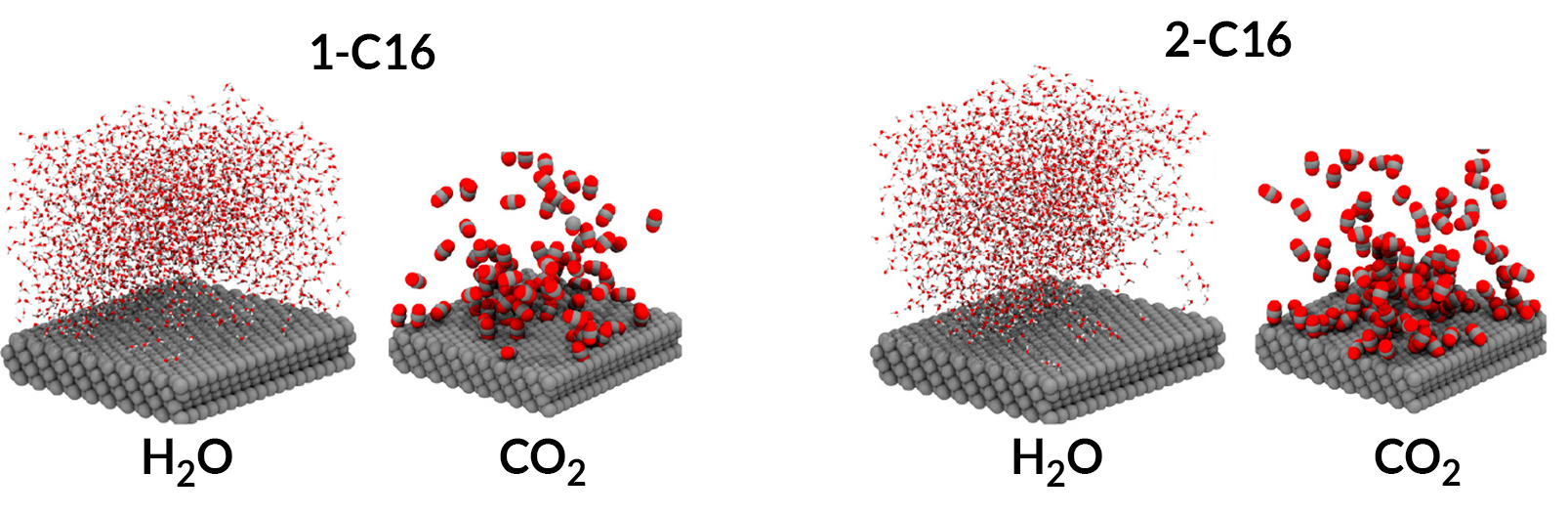
Molecular dynamics simulations revealed that the compound with the most selectivity, 2-C16, is best at concentrating CO2 near the surface. It is also hydrophobic enough to allow just the right amount of water needed to complete the reduction of CO2 to CO.
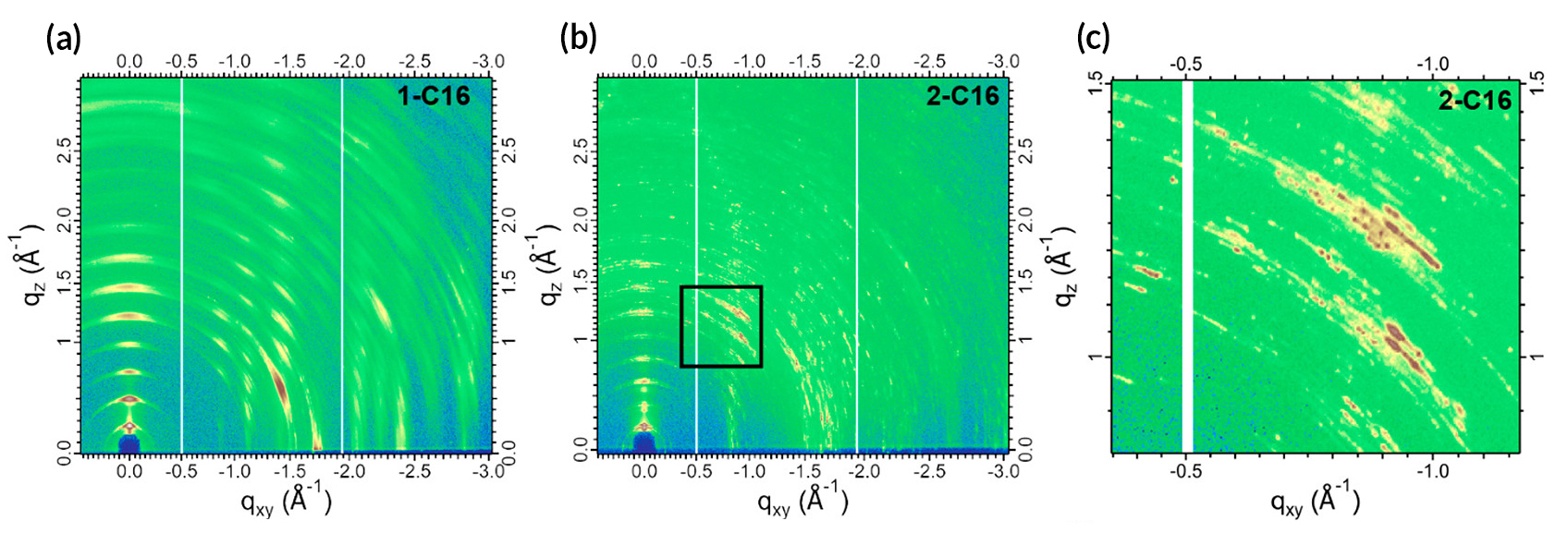
The researchers then used grazing-incidence wide-angle x-ray scattering (GIWAXS) at ALS Beamline 7.3.3 to connect function with structure. Based on the scattering data, the researchers could tell that the lattice constants and crystalline sizes were very different among the compounds. The 2-C16 data, for example, exhibited closely spaced rings with well-defined spots, indicating larger and more ordered crystallites. In contrast, the 1-C16, which performed almost (but not quite) as well, produced a streakier diffraction pattern with more widely spaced rings, indicating smaller crystallites with more random orientations.
Armed with this information, the researchers now have a head start at looking into engineering microenvironments that can replicate 2-C16’s exquisite selectivity, but in copper or a copper alloy, to convert CO2 to higher hydrocarbons in one system.
Contact: Francesca Toma
Researchers: A.K. Buckley, J. Garrison, S.W. Utan, and F.D. Toste (Berkeley Lab and UC Berkeley); T. Cheng (Soochow University, China, and Caltech); M.H. Oh (Berkeley Lab and Korea Institute of Energy Technology); G.M. Su (ALS and Berkley Lab); C. Zhu (ALS); W.A. Goddard III (Caltech); and F.M. Toma (Berkeley Lab).
Funding: U.S. Department of Energy, Office of Science, Basic Energy Sciences program (DOE BES). Operation of the ALS is supported by DOE BES.
Publication: A.K. Buckley, T. Cheng, M.H. Oh, G.M. Su, J. Garrison, S.W. Utan, C. Zhu, F.D. Toste, W.A. Goddard III, and F.M. Toma, “Approaching 100% Selectivity at Low Potential on Ag for Electrochemical CO2 Reduction to CO Using a Surface Additive,” ACS Catal. 11, 9034 (2021), doi:10.1021/acscatal.1c00830.
ALS SCIENCE HIGHLIGHT #451