Therapeutic antibodies have revolutionized the treatment of human disease; however, antibody bivalency can limit their utility against some targets due to receptor crosslinking and activation. Genentech has developed a unique one-armed antibody, onartuzumab, which is now in late-stage clinical trials in multiple cancer types. Using crystal structures obtained at ALS Beamline 5.0.2, Genentech has demonstrated the mechanism of action of this unique potentially therapeutic antibody against its target, the receptor tyrosine kinase MET.
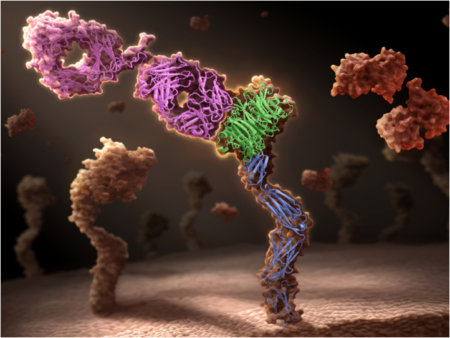
MET is activated by binding its only ligand, the hepatocyte growth factor (HGF), resulting in receptor activation and downstream signaling. HGF/MET signaling plays important roles during embryonic development and in wound healing in adults; however, in malignant cells, overactive MET leads to tumor growth, survival, and metastasis, making disruption of this interaction a promising therapeutic strategy. MET is activated by dimerization – the binding together of two MET molecules – so any process that enhances dimerization risks enhancing malignant growth rather than inhibiting it. Some traditional bivalent antibodies unfortunately do exactly that: they bind two copies of their target. Therefore, targeting MET with bivalent antibodies may mimic HGF agonism, spurring instead of halting tumor growth.
To address this problem, Genentech scientists had to look into an entirely new class of therapeutic agent: monovalent antibodies. Antibodies contain both “light chain” and “heavy chain” domains, which are assembled to generate a “Y”-shaped molecule; the bottom segment of the “Y” is known as the Fc (short for “fragment crystallizable”) region, while the arms contain what are known as the Fab (short for “fragment antigen-binding”) regions. As suggested by their names, the Fab regions bind antigens (or targets), while the Fc region provides a rigid shape and enables antibodies to have a long half-life in circulation.
To produce an antibody with just one arm, a so-called monovalent antibody, the scientists co-expressed an antibody heavy and light chain together with an Fc chain in bacteria. Efficient assembly of the monovalent antibody with minimal side products was accomplished using “knob-into-hole” mutations. These “knob-into-hole” mutations were originally invented at Genentech in the mid-1990s for assembling two different heavy chains to make bispecific antibodies (i.e. antibodies that bind two different targets). A “knob” mutation was made by replacing a small amino acid with a much larger one. This was designed to fit into a “hole” on the opposing chain constructed by replacing several amino acids with smaller ones. This technique worked to produce an antibody that binds to MET, but does not dimerize it. In addition, as the antibody contains a fully functional Fc region, the antibody has a long half-life that is necessary for therapeutic activity. The result was onartuzumab, a monovalent antibody that is designed to be a pure antagonist of HGF binding to MET.
“The ALS really helped us figure out how the antibody inhibited the function of the MET signaling pathway,” says Genentech scientist Mark Merchant. “We knew it blocked the ability of HGF to bind to MET, but we didn’t know how.”

Previous structures of MET in complex with the beta-chain of HGF had been produced by Genentech scientists in the past; however, it was not clear whether onartuzumab bound in a similar region of MET as did the beta-chain of HGF. Genentech scientists had tried multiple approaches to visualize the structure of onartuzumab with MET; however, mixing various versions of both proteins failed to generate a stable crystal that could be used in x-ray crystallography experiments. A key insight came when it was demonstrated that onartuzumab specifically blocked the high-affinity interaction between the HGF alpha-chain and MET, but not the lower-affinity interaction between the HGF beta-chain and MET. This led the researchers to attempt solving a ternary complex between the onartuzumab Fab, MET, and the HGF beta-chain. Inclusion of the HGF beta-chain helped to produce the crystal form necessary for x-ray crystallography. Through work by multiple researchers at Beamline 5.0.2, Genentech found that onartuzumab specifically binds to a region in MET required for HGF alpha-chain binding, but opposite the site where HGF beta-chain binds.
“Understanding the mechanism by which onartuzumab binds to MET helps to clarify the mechanism of action of the antibody and may provide insights into how this receptor becomes activated by HGF,” says Merchant. “Having a clear mechanism of action for an investigational molecule like this is helpful as we assess it in the lab and eventually in people.”
The molecule is currently in Phase III trials in lung cancer and is being evaluated in other cancer types as well.
“We’re very excited about onartuzumab and other antibodies made using the same knob-into-hole technology,” says Merchant. “This technology helps us to better customize our therapeutics to patient needs.”