SCIENTIFIC ACHIEVEMENT
Researchers identified the molecules that collect at the interface between a platinum electrode and an acidic electrolyte under an applied voltage.
SIGNIFICANCE AND IMPACT
Knowledge of the structure and composition of such nanometer-thin interface regions is key to understanding topics such as corrosion, geochemistry, electrocatalysis, and energy storage.
The challenge of interfaces
Material interfaces are all around us—at the ocean surface, in cell membranes, or inside any electronic device where two different conductive materials connect. Such devices are increasingly dependent on ultrathin materials to move electrons around or store energy. But there are many unanswered questions about what happens at the interfaces between these materials at the atomic scale, spurring numerous studies into the electrochemistry of buried interfaces using a variety of techniques. However, interpretation of the data obtained with these techniques often relies on assumptions that have not been substantiated at the atomic level. As a result, understanding the dynamic molecular structure of buried, functional electrochemical interfaces remains a great challenge.
What happens to platinum in sulfuric acid?
A platinum electrode immersed in sulfuric acid—an excellent electrolyte—is a model system that has been used to study electrochemistry and metal corrosion since the 1800s. A widely accepted model of what happens when you apply a voltage to this system assumes that platinum oxide (Pt–O) and platinum hydroxide (Pt–OH) form as electrons flow through the platinum. Recently, however, new studies have questioned the existence of these oxidation products. Obtaining molecular-scale details of these processes requires the ability to probe a region a few nanometers thick or less at a buried (or in this case, submerged) interface. Unfortunately, the techniques normally used to study solid surfaces require the material to be under vacuum. When another material such as a liquid is in the way, those methods no longer work.
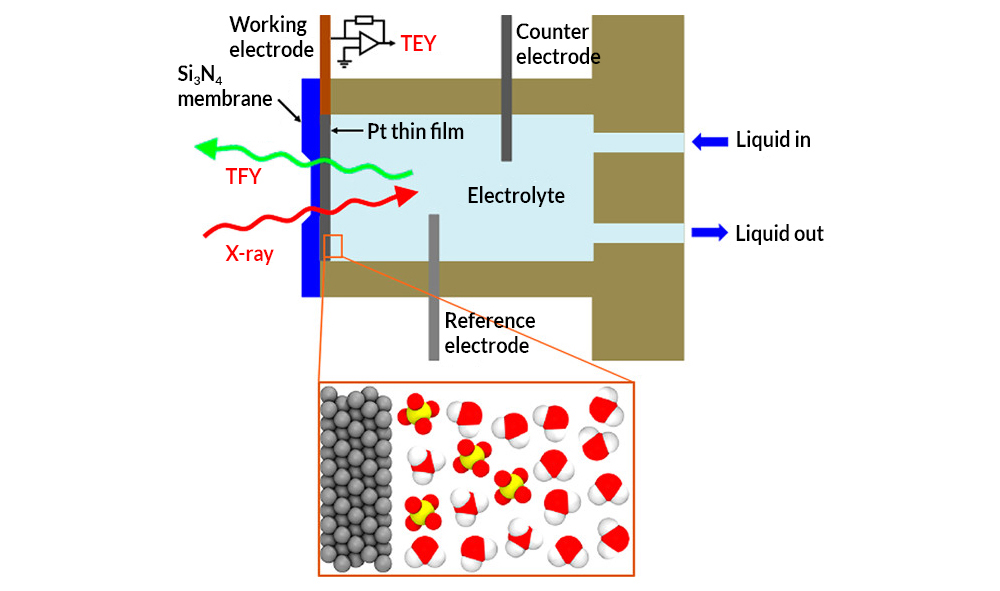
Looking up through the bottom
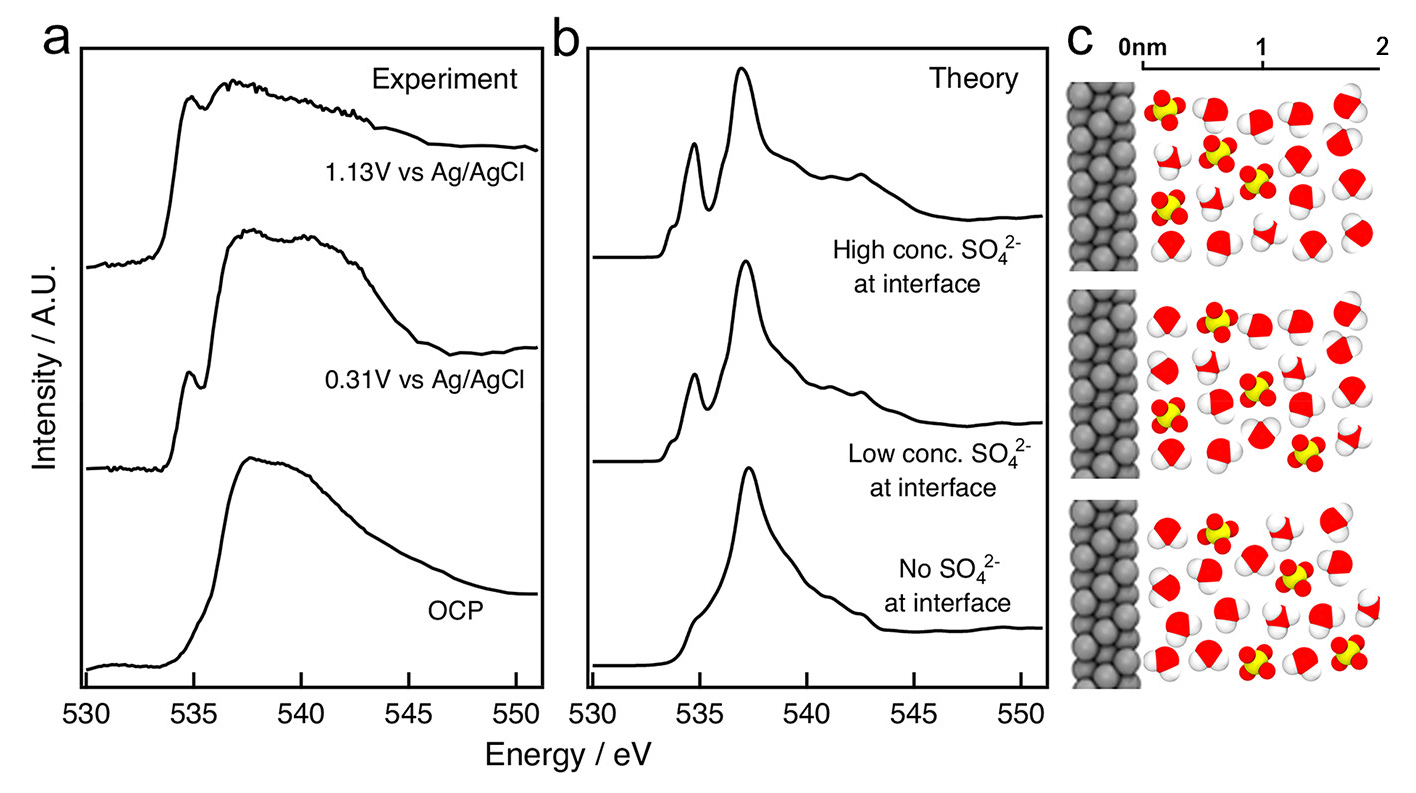
A team of researchers, led by staff from Berkeley Lab’s Materials Sciences Division, used an innovative twist on x-ray absorption spectroscopy (XAS) at ALS Beamline 8.0.1 to look up through the bottom of a very thin platinum electrode immersed in sulfuric acid. In a liquid flow cell, a thin, silicon nitride (Si3N4) membrane was used to separate the sulfuric acid electrolyte from the vacuum chamber of the x-ray source; a platinum working electrode was deposited on liquid side of the membrane. X-ray absorption was detected via bulk-sensitive total fluorescence yield (TFY) on the vacuum side and interface-sensitive total electron yield (TEY) at the platinum electrode. The TEY signal (i.e., photon-induced current) provided the spectral fingerprints of the chemical species present up to two nanometers from the interface.
Computational fingerprinting
To interpret the data, theorists at Berkeley Lab’s Molecular Foundry used the Lab’s National Energy Research Scientific Computing Center (NERSC) to run computer simulations, producing spectroscopic fingerprints of molecules that might be found at the interface. The results yielded a few surprises that challenge conventional models of electrochemical interfaces. First, the researchers found no evidence for the presence of platinum oxide despite conventional understanding. At the same time, the team measured elevated concentrations of sulfate ions—concentrations that were much higher than those found in portions of the liquid far way from the electrode.
The results demonstrate the power of surface-sensitive and element-specific XAS experiments combined with first-principles theory to probe the structure of buried, solid/liquid, functional electrochemical interfaces with molecular-scale detail, opening the way to future studies relevant to electrolysis, fuel cells, and energy storage, from batteries to supercapacitors.
Contacts: Miquel Salmeron and David Prendergast
Researchers: C.H. Wu and M.B. Salmeron (University of California, Berkeley, and Berkeley Lab); T.A. Pascal, A. Baskin, Y.-H. Lu, and D. Prendergast (Berkeley Lab); H. Wang and H.-T. Fang (Harbin Institute of Technology, China, and Berkeley Lab); Y.-S. Liu (ALS); and J.-H. Guo (ALS and University of California, Santa Cruz).
Funding: U.S. Department of Energy, Office of Science, Basic Energy Sciences Program (DOE BES). Operation of the ALS is supported by DOE BES.
Publication: C.H. Wu, T.A. Pascal, A. Baskin, H. Wang, H.-T. Fang, Y.-S. Liu, Y.-H. Lu, J.-H. Guo, D. Prendergast, and M.B. Salmeron, “Molecular-Scale Structure of Electrode–Electrolyte Interfaces: The Case of Platinum in Aqueous Sulfuric Acid,” J. Am. Chem. Soc. 140, 16237 (2018), 10.1021/jacs.8b09743.
Adapted from the Berkeley Lab news release, “Plumbing the Depths of Interfaces and Finding Buried Treasure.”
ALS SCIENCE HIGHLIGHT #391